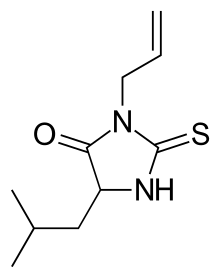
Albutoin
Albutoin is an anticonvulsant.[1][2] It was marketed in Europe as CO-ORD and Euprax by Baxter Laboratories.[1] It was evaluated by the United States Food and Drug Administration, but not approved.[3]
 | |
| Clinical data | |
|---|---|
| Other names | BAX-422Z[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H16N2OS |
| Molar mass | 212.31 g·mol−1 |
| 3D model (JSmol) | |
| |
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 24–. ISBN 978-1-4757-2085-3.
- Cereghino JJ, Brock JT, Van Meter JC, Penry JK, Smith LD, Fisher P, Ellenberg J (April 1974). "Evaluation of albutoin as an antiepileptic drug". Clinical Pharmacology and Therapeutics. 15 (4): 406–16. doi:10.1002/cpt1974154406. PMID 4206927.
- Shorvon SD (March 2009). "Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959-2009". Epilepsia. 50 Suppl 3: 93–130. doi:10.1111/j.1528-1167.2009.02042.x. PMID 19298435.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.