Alpha-beta Unsaturated carbonyl compounds
α,β-Unsaturated carbonyl compounds refers to organic compounds with the general structure (O=CR)−Cα=Cβ-R. Examples would be enones and enals. In these compounds the carbonyl group is conjugated with an alkene (hence the adjective unsaturated). Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.

Classes
- Parent α,β-Unsaturated Carbonyls
 Methyl vinyl ketone, the simplest α,β-unsaturated ketone
Methyl vinyl ketone, the simplest α,β-unsaturated ketone Acrolein, the simplest α,β-unsaturated aldehyde
Acrolein, the simplest α,β-unsaturated aldehyde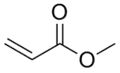 Methyl acrylate, an α,β-unsaturated ester
Methyl acrylate, an α,β-unsaturated ester Acrylamide, precursor to polyacrylamide
Acrylamide, precursor to polyacrylamide Maleic acid, an α,β-unsaturated dicarbonyl
Maleic acid, an α,β-unsaturated dicarbonyl Fumaric acid, isomeric with maleic acid
Fumaric acid, isomeric with maleic acid
α,β-Unsaturated carbonyls can be subclassified according to the nature of the carbonyl group.
α,β-unsaturated acids, esters, and amides
An α,β-unsaturated acid is a type of an α,β-unsaturated carbonyl that consists of an alkene conjugated to a carboxylic acid.[1] The simplest example is acrylic acid (CH2=CHCO2H). These compounds are prone to polymerization, giving rise to the large area of acrylate plastics. Acrylates are derived from but do not contain the acrylate group.[2] Methyl acrylate and acrylamide are commercially important examples of α,β-unsaturated esters and α,β-unsaturated amides. They also polymerize readily. Acrylic acid, its esters, and its amide derivatives feature the acryloyl group, CH2=CHC(O)-.
α,β-Unsaturated dicarbonyls are also common. The parent compounds are maleic acid and the isomeric fumaric acid. Maleic acid forms esters, an imide, and an anhydride, i.e. diethyl maleate, maleimide, and maleic anhydride. Fumaric acid, as fumarate, is an intermediate in the Krebs citric acid cycle, which is of great importance in bioenergy.
Enones
An enone is a type of an α,β-unsaturated carbonyl that consists of an alkene conjugated to a ketone.[1] The simplest enone is methyl vinyl ketone (butenone, CH2=CHCOCH3). Enones are typically produced using an aldol condensation or Knoevenagel condensation. Some commercially significant enones produced by condensations of acetone are mesityl oxide (dimer of acetone) and phorone and isophorone (trimers).[3]

In the Meyer–Schuster rearrangement, the starting compound is a propargyl alcohol. Cyclic enones can be prepared via the Pauson–Khand reaction.
Enals
An enal is a type of an α,β-unsaturated carbonyl, consisting of an alkene conjugated to a aldehyde.[1] The simplest enal is acrolein (CH2=CHCHO). Other examples include cis-3-hexenal (essence of mowed lawns) and cinnamaldehyde (essence of cinnamon).
- Other α,β-Unsaturated Carbonyls
 E-Crotonaldehyde, an enal that exists as isomers
E-Crotonaldehyde, an enal that exists as isomers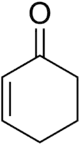 Cyclohexenone, common cyclic enone
Cyclohexenone, common cyclic enone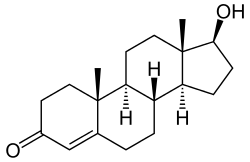 testosterone, the male sex hormone
testosterone, the male sex hormone Cinnamaldehyde, essence of cinnamon
Cinnamaldehyde, essence of cinnamon Paraquinone, a particularly electrophilic α,β-unsaturated carbonyl
Paraquinone, a particularly electrophilic α,β-unsaturated carbonyliron-tricarbonyl-2D-skeletal.png.webp) Enone complex of iron tricarbonyl
Enone complex of iron tricarbonyl
Reactions of α,β-unsaturated carbonyl
α,β-unsaturated carbonyls are electrophilic at both the carbonyl carbon as well as the β-carbon. Depending on conditions, either site is attacked by nucleophiles. Additions to the alkene are called conjugate additions. One type of conjugate addition is the Michael addition, which is used commercially in the conversion of mesityl oxide into isophorone. Owing to their extended conjugation, α,β-unsaturated carbonyls are prone to polymerization. In terms of industrial scale, polymerization dominates the use of α,β-unsaturated carbonyls. Again because of their electrophilic character, the alkene portion of α,β-unsaturated carbonyls are good dienophiles in Diels–Alder reactions. They can be further activated by Lewis acids, which bind to the carbonyl oxygen. α,β-Unsaturated carbonyls are good ligands for low-valent metal complexes, examples being Fe(bda)(CO)3 and tris(dibenzylideneacetone)dipalladium(0).
α,β-Unsaturated carbonyls are readily hydrogenated. Hydrogenation can target the carbonyl or the alkene (conjugate reduction) selectively, or both functional groups.
Enones undergo the Nazarov cyclization reaction and in the Rauhut–Currier reaction (dimerization).
Safety
Since α,β-unsaturated compounds are electrophiles and alkylating agents, many α,β-unsaturated carbonyl compounds are toxic. The endogenous scavenger compound glutathione naturally protects from toxic electrophiles in the body. Some drugs (amifostine, N-acetylcysteine) containing thiol groups may protect from such harmful alkylation.
See also
References
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Ohara, Takashi; Sato, Takahisa; Shimizu, Noboru; Prescher, Günter; Schwind, Helmut; Weiberg, Otto; Marten, Klaus; Greim, Helmut (2003). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2.
- Siegel, Hardo; Eggersdorfer, Manfred (2000). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.