Anthrone
Anthrone is a tricyclic aromatic ketone. It is used for a common cellulose assay and in the colorometric determination of carbohydrates.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
10H-Anthracen-9-one | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.813 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H10O | |
| Molar mass | 194.233 g·mol−1 |
| Appearance | White to light yellow needles |
| Melting point | 155 to 158 °C (311 to 316 °F; 428 to 431 K) |
| Boiling point | 721 °C (1,330 °F; 994 K) |
| Insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Derivatives of anthrone are used in pharmacy as laxative. They stimulate the motion of the colon and reduce water reabsorption. Some anthrone derivatives can be extracted from a variety of plants, including Rhamnus frangula, Aloe ferox, Rheum officinale, and Cassia senna.
Synthesis and reactions
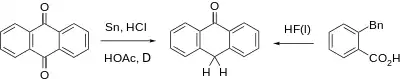
Anthrone can be prepared from anthraquinone by reduction with tin or copper.[2]
An alternative synthesis involves cyclization of o-benzylbenzoic acid induced with hydrogen fluoride.[3]
Anthrone condenses with glyoxal to give, following dehydrogenation, acedianthrone, a useful octacyclic pigment.[4]
Tautomer

Anthrone is the more stable tautomer relative to the anthrol. The tautomeric equilibrium is estimated at 100 in aqueous solution. For the two other isomeric anthrols, the tautomeric equilibrium is reversed.[5]
References
- Trevelyan, W. E.; Forrest, RS; Harrison, JS (1952). "Determination of Yeast Carbohydrates with the Anthrone Reagent". Nature. 170 (4328): 626–627. Bibcode:1952Natur.170..626T. doi:10.1038/170626a0. PMID 13002392.
- Macleod, L. C.; Allen, C. F. H. (1934). "Benzanthrone". Organic Syntheses. 14: 4. doi:10.15227/orgsyn.014.0004.
- Fieser, Louis F.; Hershberg, E. B. (May 1939). "Inter- and Intramolecular Acylations with Hydrogen Fluoride". Journal of the American Chemical Society. 61 (5): 1272–1281. doi:10.1021/ja01874a079.
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. (2005). "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- Ośmiałowski, Borys; Raczyńska, Ewa D.; Krygowski, Tadeusz M. (2006). "Tautomeric Equilibria and Pi Electron Delocalization for Some Monohydroxyarenes Quantum Chemical Studies". The Journal of Organic Chemistry. 71 (10): 3727–3736. doi:10.1021/jo052615q. PMID 16674042.