Atmospheric-pressure chemical ionization
Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa),[1][2] commonly coupled with high-performance liquid chromatography (HPLC).[3] APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray.[4] The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da.[5] The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.[6]

Instrument structure
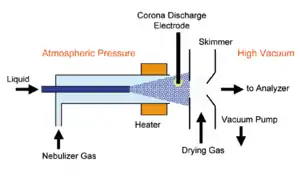
A typical APCI usually consists of three main parts: a nebulizer probe which can be heated to 350-500 °C, an ionization region with a corona discharge needle, and an ion-transfer region under intermediate pressure.[5] The analyte in solution is introduced from a direct inlet probe or a liquid chromatography (LC) eluate into a pneumatic nebulizer with a flow rate 0.2–2.0mL/min. In the heated nebulizer, the analyte coaxially flows with nebulizer N2 gas to produce a mist of fine droplets. By the combination effects of heat and gas flow, the emerged mist is converted into a gas stream. Once the gas stream arrives in the ionization region under atmospheric pressure, molecules are ionized at corona discharge which is 2 to 3 kV potential different to the exit counter-electrode.[4] Sample ions then pass through a small orifice skimmer into the ion-transfer region. Ions may be transported through additional skimmer or ion-focusing lenses into a mass analyzer for subsequent mass analysis.
Ionization mechanism
Ionization in the gas phase by APCI follows the sequences: sample in solution, sample vapor, and sample ions. The effluent from the HPLC is evaporated completely. The mixture of solvent and sample vapor is then ionized by ion-molecule reaction.[7]
The ionization can either be carried out in positive or negative ionization mode. In the positive mode, the relative proton affinities of the reactant ions and the gaseous analyte molecules allow either proton transfer or adduction of reactant gas ions to produce the ions [M+H]+ of the molecular species.[4] In the negative mode, [M−H]− ions are produced by either proton abstraction, or [M+X]− ions are produced by anion attachment. Most work on the APCI-MS analysis has been in positive mode.
In the positive mode, when the discharge current of corona discharge is 1-5 μA on the nebulized solvent, N2 gas molecules are excited and ionized, which produce N4+*. The evaporated mobile phase of LC acts as the ionization gas and reactant ions. If water is the only solvent in the evaporated mobile phase, the excited nitrogen molecular ions N4+* would react with H2O molecules to produce water cluster ions H+(H2O)n.[8] Then, analyte molecules M are protonated by the water cluster ions. Finally, the ionization products MH+(H2O)m transfer out from the atmospheric-pressure ion source. Declustering (removal of water molecules from the protonated analyte molecule) of MH+(H2O)m takes place at the high vacuum of the mass analyzer.[2] The analyte molecule ions detected by MS are [M+H]+. The chemical reactions of ionization process are shown below.
Primary and secondary reagent ion formation in a nitrogen atmosphere in the presence of water:[9][2]
- N2 + e → N2+ + 2e
- N2+* + 2N2 → N4+* + N2
- N4+ + H2O → H2O+ + 2N2
- H2O+ + H2O → H3O+ + OH•
- H3O+ + H2O + N2 → H+(H2O)2 + N2
- H+(H2O)n-1 + H2O + N2 → H+(H2O)n + N2
Ionization of product ions:[2]
- H+(H2O)n + M → MH+(H2O)m + (n-m)H2O
Declustering in the high vacuum of the mass analyzer:[2]
- MH+(H2O)m → MH+ + mH2O
If the mobile phase contains solvents with a higher proton affinity than water, proton-transfer reactions take place that lead to protonated the solvent with higher proton affinity. For example, when methanol solvent is present, the cluster solvent ions would be CH3OH2+(H2O)n(CH3OH)m.[2] Fragmentation does not normally occur inside the APCI source. If a fragment ion of a sample is observed, thermal degradation has taken place by the heated nebulizer interface, followed by the ionization of the decomposition products.
In a major distinction from chemical ionization, the electrons needed for the primary ionization are not produced by a heated filament, as a heated filament cannot be used under atmospheric pressure conditions. Instead, the ionization must occur using either corona discharges or β- particle emitters, which are both electron sources capable of handling the presence of corrosive or oxidizing gases.[4]
History
The first atmospheric pressure ionization source was developed by Horning, Carroll and their co-works in the 1970s at the Baylor College of Medicine (Houston, TX).[10] Initially, 63Ni foil was used as a source of electrons to perform ionization. Latterly in 1975, corona discharge electrode was developed, which had a larger dynamic response range.[11] APCI with the corona discharge electrode became the model for modern commercially available APCI interfaces.[9]
APCI was applied to GC/MS[10] and LC/MS[12] also by Horning's group in 1975. Analyte in LC eluate was vaporized and ionized in a heated block. High sensitivity and simple mass spectra were obtained through this application.[12] In the later decades, the coupling of APCI with LC/MS became famous and caught a lot attention.[3] The introduction of APCI and LC-MS had expanded dramatically the role of mass spectrometry in the pharmaceutical industry in the area of drug development. The sensitivity of APCI combined with the sensitivity and specificity of LC/MS and liquid chromatography-tandem mass spectrometry (LC-MS/MS) makes it the method of choice for the quantification of drugs and drug metabolites.[13]
Advantages
Ionization of the substrate is very efficient as it occurs at atmospheric pressure, and thus has a high collision frequency. Additionally, APCI considerably reduces the thermal decomposition of the analyte because of the rapid desolvation and vaporization of the droplets in the initial stages of the ionization.[4] This combination of factors most typically results in the production of ions of the molecular species with fewer fragmentations than many other ionization methods, making it a soft ionization method.[14]
Another advantage to using APCI over other ionization methods is that it allows for the high flow rates typical of standard bore HPLC (0.2-2.0mL/min) to be used directly, often without diverting the larger fraction of volume to waste. Additionally, APCI can often be performed in a modified ESI source.[15] The ionization occurs in the gas phase, unlike ESI, where the ionization occurs in the liquid phase. A potential advantage of APCI is that it is possible to use a nonpolar solvent as a mobile phase solution, instead of a polar solvent, because the solvent and molecules of interest are converted to a gaseous state before reaching the corona discharge needle. Because of APCI involves a gas-phase chemistry, there is no need to use special conditions such as solvents, conductivity, pH for LC. APCI appeared to be more versatile LC/MS interface and more compatible with reversed-phase LC than ESI.[14]
Application
APCI is suited for thermal stable samples with low to medium (less than 1500Da) molecular weight, and medium to high polarity. The application area of APCI is the analysis of drugs, nonpolar lipids, natural compounds, pesticides and various organic compounds, but limited to the analysis of biopolymers, organometallics, ionic compounds and other labile analytes.[16]
See also
References
- Carroll, D. I.; Dzidic, I.; Stillwell, R. N.; Horning, M. G.; Horning, E. C. (1974). "Subpicogram detection system for gas phase analysis based upon atmospheric pressure ionization (API) mass spectrometry". Analytical Chemistry. 46 (6): 706–710. doi:10.1021/ac60342a009. ISSN 0003-2700.
- Niessen, Wilfried (2006). Liquid Chromatography Mass spectrometry. 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33478: CRC Press, Taylor and Francis Group. pp. 249–250. ISBN 978-0585138503.CS1 maint: location (link)
- Thomson, Bruce A. (1998-03-01). "Atmospheric pressure ionization and liquid chromatography/mass spectrometry—together at last". Journal of the American Society for Mass Spectrometry. 9 (3): 187–193. doi:10.1016/S1044-0305(97)00285-7. ISSN 1044-0305.
- Edmond de Hoffmann; Vincent Stroobant (22 October 2007). Mass Spectrometry: Principles and Applications. Wiley. ISBN 978-0-470-51213-5.
- Dass, Chhabil (2007). Fundamentals of Contemporary Mass Spectrometry. John Wiley & Sons, Inc. p. 47. ISBN 978-0-471-68229-5.
- Bruins, A. P. (1991). "Mass spectrometry with ion sources operating at atmospheric pressure". Mass Spectrometry Reviews. 10 (1): 53–77. Bibcode:1991MSRv...10...53B. doi:10.1002/mas.1280100104. ISSN 0277-7037.
- AP, BRUINS (1994-01-01). "ATMOSPHERIC-PRESSURE-IONIZATION MASS-SPECTROMETRY .2. APPLICATIONS IN PHARMACY, BIOCHEMISTRY AND GENERAL-CHEMISTRY". TrAC Trends in Analytical Chemistry. 13 (2). ISSN 0165-9936.
- Gates, Paul. University of Bristol, Department of Chemistry, "Atmospheric Pressure Chemical Ionization." Last modified 2004. Accessed November 22, 2013. "Archived copy". Archived from the original on 2013-11-26. Retrieved 2013-12-06.CS1 maint: archived copy as title (link).
- Byrdwell, William Craig (2001-04-01). "Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids". Lipids. 36 (4): 327–346. doi:10.1007/s11745-001-0725-5. ISSN 0024-4201. PMID 11383683.
- Horning, E. C.; Horning, M. G.; Carroll, D. I.; Dzidic, I.; Stillwell, R. N. (1973-05-01). "New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure". Analytical Chemistry. 45 (6): 936–943. doi:10.1021/ac60328a035. ISSN 0003-2700.
- Carroll, D. I.; Dzidic, I.; Stillwell, R. N.; Haegele, K. D.; Horning, E. C. (1975-12-01). "Atmospheric pressure ionization mass spectrometry. Corona discharge ion source for use in a liquid chromatograph-mass spectrometer-computer analytical system". Analytical Chemistry. 47 (14): 2369–2373. doi:10.1021/ac60364a031. ISSN 0003-2700.
- Horning, E. C.; Carroll, D. I.; Dzidic, I.; Haegele, K. D.; Horning, M. G.; Stillwell, R. N. (1974-11-01). "Atmospheric pressure ionization (API) mass spectrometry. Solvent-mediated ionization of samples introduced in solution and in a liquid chromatograph effluent stream". Journal of Chromatographic Science. 12 (11): 725–729. doi:10.1093/chromsci/12.11.725. ISSN 0021-9665. PMID 4424244.
- Taylor, Lester C. E.; Johnson, Robert L.; Raso, Roberto (1995-05-01). "Open access atmospheric pressure chemical ionization Mass spectrometry for routine sample analysis". Journal of the American Society for Mass Spectrometry. 6 (5): 387–393. doi:10.1016/1044-0305(94)00124-1. ISSN 1044-0305. PMID 24214220.
- Zaikin, Vladimir G.; Halket, John M. (2017). "Derivatization in Mass Spectrometry—8. Soft Ionization Mass Spectrometry of Small Molecules". European Journal of Mass Spectrometry. 12 (2): 79–115. doi:10.1255/ejms.798. ISSN 1469-0667. PMID 16723751.
- Holčapek, Michal; Jirásko, Robert; Lísa, Miroslav (2012). "Recent developments in liquid chromatography–mass spectrometry and related techniques". Journal of Chromatography A. 1259: 3–15. doi:10.1016/j.chroma.2012.08.072. ISSN 0021-9673. PMID 22959775.
- Holčapek, Michal; Jirásko, Robert; Lísa, Miroslav (2010-06-18). "Basic rules for the interpretation of atmospheric pressure ionization mass spectra of small molecules". Journal of Chromatography A. Mass Spectrometry: Innovation and Application. Part VI. 1217 (25): 3908–3921. doi:10.1016/j.chroma.2010.02.049. PMID 20303090.