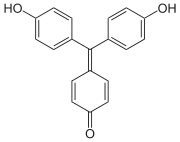
Aurin
Aurin (C.I. 43800), sometimes named rosolic acid or corallin is an organic compound, forming yellowish or deep-red crystals with greenish metallic luster. It is practically insoluble in water, freely soluble in alcohol. It is soluble in strong acids to form yellow solution, or in aqueous alkalis to form carmine red solutions.
| Aurin (pH indicator) | ||
| below pH 5.0 | above pH 6.8 | |
| 5.0 | ⇌ | 6.8 |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-[Bis(p-hydroxyphenyl)methylene]-2,5-cyclohexadien-1-one | |
| Other names
Aurin, corallin, p-rosolic acid, C.I. 43800 | |
| Identifiers | |
3D model (JSmol) |
|
| 2055205 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.129 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H14O3 | |
| Molar mass | 290.318 g·mol−1 |
| Appearance | see text |
| Density | 1.283 g/cm3 |
| Melting point | 308 °C (586 °F; 581 K) (decomposes) |
| Insoluble | |
| UV-vis (λmax) | 482 nm[1] |
| -161.4·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |  [1] [1] |
| GHS Signal word | Danger |
| H315, H319, H335[1] | |
| P261, P305+351+338[1] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Due to this behaviour it can be used as pH indicator with pH transition range 5.0 - 6.8. It is used as an intermediate in manufacturing of dyes.
Synthesis
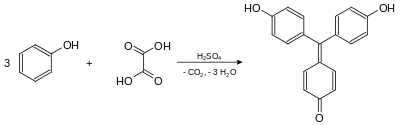
Aurin was first prepared in 1834 by the German chemist Friedlieb Ferdinand Runge, who obtained it by distilling coal tar. He named it Rosölsäure or Rosaölsäure (red oil acid).[2][3] In 1861, the German chemists Hermann Kolbe and Rudolf Schmitt presented the synthesis of aurin by heating oxalic acid and creosote (which contains phenol) in the presence of concentrated sulfuric acid.[4] (Gradually, chemists realized that commercial aurin was not a pure compound, but was actually a mixture of similar compounds.[5][6])
Aurin is formed by heating of phenol and oxalic acid in concentrated sulfuric acid.
Safety
Causes eye, skin, and respiratory tract irritation. Avoid ingestion and/ or inhalation.
References
- Sigma-Aldrich Co., p-Rosolic acid. Retrieved on 2014-05-06.
- Runge, F. F. (1834). "Ueber einige Produkte der Steinkohlendestillation" [On some products of coal tar distillation]. Annalen der Physik und Chemie (in German). 31: 65–78. ; see p. 70.
- In 1859, the German-English chemist Hugo Müller (de) (1833–1915) found that rosolic acid could be produced simply by mixing phenol and calcium carbonate and then exposing the mixture to air for a prolonged time. Müller, Hugo (1859). "Note on rosolic acid". Quarterly Journal of the Chemical Society. 11 (1): 1–5.
- Kolbe, H.; Schmitt, R. (1861). "Rother Farbstoff aus dem Kreosot" [Red dye from creosote]. Annalen der Chemie (in German). 119: 169–172.
- See the papers of the English chemist Richard S. Dale and the German-English chemist Carl Schorlemmer and of the German chemist Heinrich Fresenius (de) (1847–1920).
- Dale, R.S.; Schorlemmer, C. (1871). "On aurin". Journal of the Chemical Society. 24: 466–467.
- Dale, R.S.; Schorlemmer, C. (1872). "On aurin". Journal of the Chemical Society. 25: 74–75.
- Dale, R.S.; Schorlemmer, C. (1873). "On aurin". Journal of the Chemical Society. 26: 434–444.
- Dale, R.S.; Schorlemmer, C. (1879). "On aurin, part II". Journal of the Chemical Society. 35: 148–159.
- Fresenius, H. (1871). "Ueber Rosolsäure" [On rosolic acid]. Journal für praktische Chemie. 2nd series (in German). 3: 477–480.
- A review of findings about aurin up to 1872 appeared in: Fresenius, H. (1872). "Ueber das Corallin" [On corallin]. Journal für praktische Chemie. 2nd series (in German). 5 (4): 184–191.
- Fresenius, H. (1872). "Corallin". Journal of the Chemical Society. 25: 705–706.
- Thorpe, Thomas Edward, ed., A Dictionary of Applied Chemistry, 2nd ed. (London, England: Longmans, Green, and Co., 1913), vol. 5, "Triphenylmethane colouring matters," p. 551. From p. 551: "The researches of Dale and Schorlemmer, Zulkowski, and others have shown that common aurin consists of a mixture of a number of substances, which, in a pure state, are well crystallised."