Caldoramide
Caldoramide is a pentapeptide[1] isolated from the cyanobacteria Caldora penicillata.[2] It has cytotoxic effects on cancer cells and has been the subject of extensive oncological research.[3] It is structurally analogous to belamide A and dolastatin 15. Its appearance is that of a powdery, white, substance.[3]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C37H59N5O6 | |
| Molar mass | 669.908 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
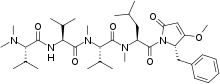
Structure
The N-terminus for Caldoramide is N,Ndimethylvaline which is attached to a valine which is attached to an N-Me-valine connected to an N-Me-isoleucine which is attached to the C-terminus. The molecule can also be written as N,N-diMe-Val-Val-N-Me-ValN-Me-Ile-3-O-Me-4-benzylpyrrolinone.[3]
Extraction
Freeze-dried samples of Caldora penicillata had EtOAc−MeOH and H2O−EtOH applied to them in order to extract Caldoramide. The extractswere partitioned with n-BuOH and H2O and then fractions were taken based on solubility in either EtOAc or BuOH. Caldoramide was extracted from the BuOH soluble fraction.[3]
Pharmacological activity
Caldoramide has been found to be cytotoxic against HCT116 colorectal cancer cell lines.[3]
References
- Wunder A, Rothemund M, Schobert R (2018). "Synthesis and anticancer activity of the proposed structure of caldoramide, an N-peptidyltetramate from the cyanobacterium Caldora penicillata". Tetrahedron. doi:10.1016/j.tet.2018.04.004.
- Iwasaki A, Tadenuma T, Sumimoto S, Shiota I, Matsubara T, Saito-Nakano Y, et al. (November 2018). "Hoshinoamides A and B, Acyclic Lipopeptides from the Marine Cyanobacterium Caldora penicillata". Journal of Natural Products. 81 (11): 2545–2552. doi:10.1021/acs.jnatprod.8b00643. PMID 30387355.
- Gunasekera SP, Imperial L, Garst C, Ratnayake R, Dang LH, Paul VJ, Luesch H (July 2016). "Caldoramide, a Modified Pentapeptide from the Marine Cyanobacterium Caldora penicillata". Journal of Natural Products. 79 (7): 1867–71. doi:10.1021/acs.jnatprod.6b00203. PMC 5215049. PMID 27380142.