Chromium(III) acetate
Chromium(III) acetate, commonly known as basic chromium acetate,[1] describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.
 | |
| Names | |
|---|---|
| IUPAC name
Chromium(III) acetate hydrate | |
| Other names
chromic acetate, chromium triacetate, chromium(III) ethanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.646 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H36ClCr3O22 | |
| Molar mass | 723.84 g·mol−1 |
| Appearance | grayish-green to blueish-green solid |
| Density | 1.662 g/cm3 |
| -5104.0·10−6 cm3/mol | |
| Structure | |
| octahedral | |
| Related compounds | |
Related compounds |
Manganese(III) acetate Iron(III) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
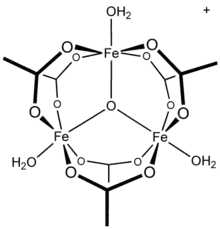
Salts of basic chromium acetate has long attracted interest because of its distinctive structure, which features octahedral Cr(III) centers, a triply bridging oxo ligand, six acetate ligands, and three aquo ligands.[1] The same structure is shared with basic iron acetate and basic manganese acetate.[1][2] Little evidence exists for a simple chromium(III) acetate, i.e. lacking the oxo ligand.[3] Chromium(III) acetate is a blue/grey-green powder, which is soluble in water. It is still[2] prepared according to the original procedure from 1909.[4]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Kurt J. Schenk, Hans U. Güdel (1982). "Low-temperature structural and spectroscopic properties of [Cr3O(CH3COO)6(H2O)3]Cl.6H2O". Inorg. Chem. 21 (6): 2253–2256. doi:10.1021/ic00136a025.CS1 maint: uses authors parameter (link)
- Erre, Liliana Strinna; Micera, Giovanni; Glowiak, Tadeusz; Kozlowski, Henry (April 1997). "Chromium (III) Acetate, Chromium (III) Acetate Hydroxide, or µ3-Oxo-esakis-(µ2-acetato-O,O') - triaqua-trichromium (III) Acetate? Determining the Structure of a Complex Compound by Analytical and Spectroscopic Methods". Journal of Chemical Education. 74 (4): 432. doi:10.1021/ed074p432.
- R. Weinland P. Dinkelacker (1909). "Über Salze einer Hexaacetato(formiato)‐trichrombase. II". Berichte der Deutschen Chemischen Gesellschaft. 42 (3): 2997–3018. doi:10.1002/cber.19090420318.CS1 maint: uses authors parameter (link)
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||