Diroximel fumarate
Diroximel fumarate, sold under the brand name Vumerity, is a medication used for the treatment of relapsing forms of multiple sclerosis (MS).[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Vumerity |
| Other names | ALKS-8700 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620002 |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
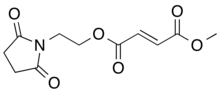
| Formula | C11H13NO6 |
| Molar mass | 255.226 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Diroximel fumarate was approved for medical use in the United States in October 2019.[2]
References
- "Vumerity- diroximel fumarate capsule". DailyMed. Retrieved 1 February 2021.
- "Drug Approval Package: Vumerity". U.S. Food and Drug Administration (FDA). 21 April 2020. Retrieved 1 February 2021.
External links
- "Diroximel fumarate". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.