Lenoir cycle
The Lenoir cycle is an idealized thermodynamic cycle often used to model a pulse jet engine. It is based on the operation of an engine patented by Jean Joseph Etienne Lenoir in 1860. This engine is often thought of as the first commercially produced internal combustion engine. The absence of any compression process in the design leads to lower thermal efficiency than the more well known Otto cycle and Diesel cycle.
| Thermodynamics |
|---|
 |
|
The cycle
In the cycle, an ideal gas undergoes[1][2]
- 1–2: Constant volume (isochoric) heat addition;
- 2–3: Isentropic expansion;
- 3–1: Constant pressure (isobaric) heat rejection.
The expansion process is isentropic and hence involves no heat interaction. Energy is absorbed as heat during the isochoric heating and rejected as work during the isentropic expansion. Waste heat is rejected during the isobaric cooling which consumes some work.
Constant volume heat addition (1–2)
In the ideal gas version of the traditional Lenoir cycle, the first stage (1–2) involves the addition of heat in a constant volume manner. This results in the following for the first law of thermodynamics:
There is no work during the process because the volume is held constant:
and from the definition of constant volume specific heats for an ideal gas:
Where R is the ideal gas constant and γ is the ratio of specific heats (approximately 287 J/(kg·K) and 1.4 for air respectively). The pressure after the heat addition can be calculated from the ideal gas law:
Isentropic expansion (2–3)
The second stage (2–3) involves a reversible adiabatic expansion of the fluid back to its original pressure. It can be determined for an isentropic process that the second law of thermodynamics results in the following:
Where for this specific cycle. The first law of thermodynamics results in the following for this expansion process: because for an adiabatic process:
Constant pressure heat rejection (3–1)
The final stage (3–1) involves a constant pressure heat rejection back to the original state. From the first law of thermodynamics we find: .
From the definition of work: , we recover the following for the heat rejected during this process: .
As a result, we can determine the heat rejected as follows: . For an ideal gas, .
Efficiency
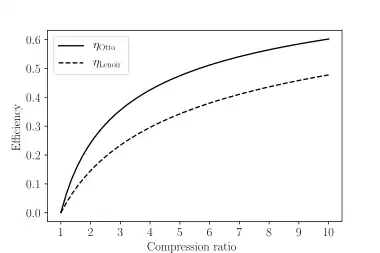
The overall efficiency of the cycle is determined by the total work over the heat input, which for a Lenoir cycle equals
Note that we gain work during the expansion process but lose some during the heat rejection process. Alternatively, the first law of thermodynamics can be used to put the efficiency in terms of the heat absorbed and heat rejected,
Utilizing that, for the isobaric process, T3/T1 = V3/V1, and for the adiabatic process, T2/T3 = (V3/V1)γ−1, the efficiency can be put in terms of the compression ratio,
where r = V3/V1 is defined to be > 1. Comparing this to the Otto cycle's efficiency graphically, it can be seen that the Otto cycle is more efficient at a given compression ratio. Alternatively, using the relationship given by process 2–3, the efficiency can be put in terms of rp = p2/p3, the pressure ratio,[2]
Cycle diagrams
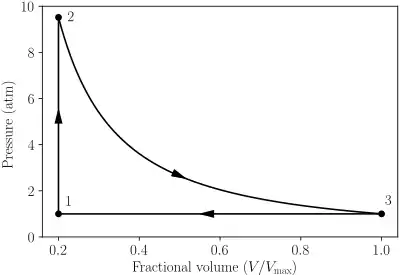 PV diagram of the Lenoir cycle |
 TS diagram of the Lenoir cycle |
References
- V. Ganesan (7 July 2008). Internal Combustion Engines. Tata McGraw-Hill Publishing Company. ISBN 9780070648173. Retrieved 2013-04-04.
- Gupta, H. N. (2013-05-19). Fundamentals of Internal Combustion Engines (2nd ed.). PHI Learning Pvt. Ltd. p. 60. ISBN 9788120346802. Retrieved 2020-05-19.