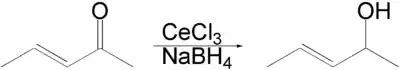Luche reduction
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols with sodium borohydride (NaBH4) and lanthanide chlorides, mainly cerium(III) chloride (CeCl3), in methanol or ethanol.[1][2][3] The Luche reduction can be conducted chemoselectively toward ketone in the presence of aldehyde or toward α,β-unsaturated ketone in the presence of non-conjugated ketone.[4]
| Luche reduction | |
|---|---|
| Named after | Jean Louis Luche |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | luche-reduction |
| RSC ontology ID | RXNO:0000286 |
An enone forms an allylic alcohol in a 1,2-addition, and the competing conjugate 1,4-addition is suppressed.
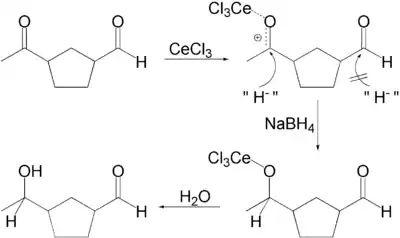
The selectivity can be explained in terms of the HSAB theory: carbonyl groups require hard nucleophiles for 1,2-addition. The hardness of the borohydride is increased by replacing hydride groups with alkoxide groups, a reaction catalyzed by the cerium salt by increasing the electrophilicity of the carbonyl group. This is selective for ketones because they are more Lewis basic.
In one application, a ketone is selectively reduced in the presence of an aldehyde. Actually, in the presence of methanol as solvent, the aldehyde forms methoxy acetal that is inactive in the reducing conditions.
References
- Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press. ISBN 0-12-429785-4.
- Luche, J.-L. (1978). "Lanthanides in Organic Chemistry. 1. Selective 1,2 Reductions of Conjugated Ketones". J. Am. Chem. Soc. 100 (7): 2226–2227. doi:10.1021/ja00475a040.
- Luche, J.-L.; Rodriguez-Hahn, L.; Crabbé, P. (1978). "Reduction of Natural Enones in the Presence of Cerium Trichloride". J. Chem. Soc., Chem. Commun. (14): 601–602. doi:10.1039/C39780000601.
- Gemal, A. L.; Luche, J.-L. (1981). "Lanthanoids in Organic Synthesis. 6. The Reduction of α-Enones by Sodium Borohydride in the Presence of Lanthanoid Chlorides: Synthetic and Mechanistic Aspects". J. Am. Chem. Soc. 103 (18): 5454–5459. doi:10.1021/ja00408a029.