Phenyl(trichloromethyl)mercury
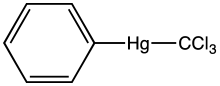
Phenyl(trichloromethyl)mercury is an organomercury compound with the formula C6H5HgCCl3. It is a white solid that is soluble in organic solvents. The compound is used as a source of dichlorocarbene, e.g. in cyclopropanation reactions, illustrated with tetrachloroethylene as a substrate, the product being hexachlorocyclopropane:[1]
- C6H5HgCCl3 → C6H5HgCl + CCl2
- CCl2 + Cl2C=CCl2 → C3Cl6
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C7H5Cl3Hg | |
| Molar mass | 396.06 g·mol−1 |
| Appearance | white solid |
| Melting point | 117–118 °C (243–244 °F; 390–391 K) |
| Hazards | |
| Main hazards | toxicity |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H300, H310, H330, H373, H400, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound is prepared by treating phenylmercuric chloride with sources of dichlorocarbene. These include the base/haloform reaction and thermolysis of sodium trichloroacetate:[2][3]
- NaO2CCCl3 + C6H5HgCl → C6H5HgCCl3 + NaCl + CO2
Related compounds
Closely related compounds include phenyl(bromodichloromethyl)mercury (CAS registry number 3294-58-4) and phenyl(tribromomethyl)mercury (CAS registry number 3294-60-8).[4] According to X-ray crystallography, the former has nearly linear coordination geometry at mercury, with a C-Hg-C angle of 179° and Hg-C distances of 2.047 Å.[5]
Also known is bis(trichloromethyl)mercury, Hg(CCl3)2.
References
- José Barluenga, Miguel Tomás, José M. González (2001). "Phenyl(trichloromethyl)mercury". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp141. ISBN 0471936235.CS1 maint: uses authors parameter (link)
- Ted J. Logan (1966). "Phenyl(trichloromethyl)mercury". Organic Syntheses. 46: 969. doi:10.15227/orgsyn.046.0098.
- Seyferth, D.; Lambert, R. L. (1969). "Halomethyl-metal compounds XX. An improved synthesis of phenyl(trihalomethyl)mercury compounds". Journal of Organometallic Chemistry. 16: 21–26. doi:10.1016/S0022-328X(00)81631-9.CS1 maint: uses authors parameter (link)
- Shipman, Michael (2001). "Phenyl(tribromomethyl)mercury". Encyclopedia of Reagents for Organic Synthesis. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp140. ISBN 0471936235.
- R. E. Bachman, B. R. Maughon, D. J. McCord, K. H. Whitmire, W. E. Billups (1995). "Bromodichloromethyl)phenylmercury". Acta Crystallogr. C. 51 (10): 2033–2035. doi:10.1107/S0108270195004501.CS1 maint: uses authors parameter (link)