Restenosis
Restenosis is the recurrence of stenosis, a narrowing of a blood vessel, leading to restricted blood flow. Restenosis usually pertains to an artery or other large blood vessel that has become narrowed, received treatment to clear the blockage and subsequently become renarrowed. This is usually restenosis of an artery, or other blood vessel, or possibly a vessel within an organ.
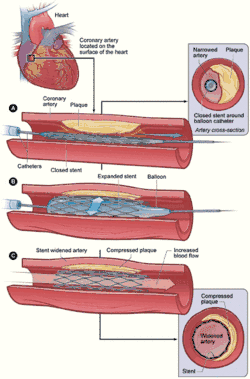
Restenosis is a common adverse event of endovascular procedures. Procedures frequently used to treat the vascular damage from atherosclerosis and related narrowing and renarrowing (restenosis) of blood vessels include vascular surgery, cardiac surgery, and angioplasty.[1]
When a stent is used and restenosis occurs, this is called in-stent restenosis or ISR.[2] If it occurs following balloon angioplasty, this is called post-angioplasty restenosis or PARS. The diagnostic threshold for restenosis in both ISR or PARS is ≥50% stenosis.[3]
If restenosis occurs after a procedure, follow-up imaging is not the only way to initially detect compromised blood flow. Symptoms may also suggest or signal restenosis, but this should be confirmed by imaging. For instance, a coronary stent patient who develops restenosis may experience recurrent chest pain (angina) or suffer from a minor or major heart attack (myocardial infarction), though they may not report it. This is why it is important that a patient comply with follow-up screenings and the clinician follows through with a thorough clinical assessment. But it is also important to note that not all cases of restenosis lead to clinical symptoms, nor are they asymptomatic.[3]
Causes
Surgery to widen or unblock a blood vessel usually has a long-lasting beneficial effect for the patient. However, in some cases, the procedure itself can cause further narrowing of the vessel, or restenosis. Angioplasty, also called percutaneous transluminal coronary angioplasty (PTCA), is commonly used to treat blockages of the coronary or peripheral arteries (such as in the limbs). The balloon inserted into the narrowing ‘smashes’ the cholesterol plaques (atherosclerosis) against the artery walls, thus widening the size of the lumen and increasing blood flow. However the action damages the artery walls, and they respond by using physiological mechanisms to repair the damage. (See physiology below.) [4]
A stent is a mesh, tube-like structure often used in conjunction with angioplasty to permanently hold open an artery, allowing for unrestricted blood flow, or to support a weakness in the artery wall called an aneurysm. The artery can react to the stent, perceive it as a foreign body, and respond by mounting an immune system response which leads to further narrowing near or inside the stent.
Physiology
Damage to the blood vessel wall by angioplasty triggers physiological response that can be divided into two stages. The first stage that occurs immediately after tissue trauma, is thrombosis. A blood clot forms at the site of damage and further hinders blood flow. This is accompanied by an inflammatory immune response.
The second stage tends to occur 3–6 months after surgery and is the result of proliferation of cells in the media, a smooth muscle wall in the vessel. This is also known as Neointimal Hyperplasia (NIHA).[5]
Diagnosis
Imaging
Vessel restenosis is typically detected by angiography, but can also be detected by duplex ultrasound and other imaging techniques.[6]
As "late loss"
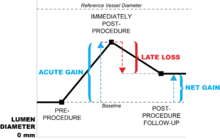
Late loss is synonymous with restenosis, and literally means loss of the lumen after a procedure intended to open the vessel. It measures either the percent (relative) or absolute change in minimum luminal diameter (MLD) over the months following a vascular procedure, such as the implantation of a stent-graft. Late loss is one metric that is useful in determining the effectiveness of vascular interventions in clinical trials for either an individual patient or a group of patients.
But late loss is only part of the terminology in describing the outcomes of vascular interventions. For instance, the implantation of a stent-graft will first provide an acute gain in lumen diameter. In other words, there is an immediate gain in lumen size because the implanted stent opens up the vessel. However, over time, the body's inflammatory immune response (described below in the "Causes" section) reacts to the stent-graft via smooth muscle proliferation, etc., which literally pushes the stent-graft back, narrowing the vessel and losing at least a percentage of what was previously gained, or late loss.
The net gain of lumen diameter is the difference between acute gain and late loss, and is a measure of stent-graft effectiveness.[7]
Percent diameter restenosis
Percent diameter restenosis (or just percent diameter stenosis) is a measure observed in individual patients and is typically calculated as the difference between the minimal (or minimum) luminal diameter (MLD) from the target reference vessel diameter (RVD), divided by the RVD, and multiplied by 100 to get the percentage of stenosis. It is an important measure needed to calculate binary restenosis (see Binary Restenosis section below). The RVD is typically calculated by averaging the MLD of the healthy part of the vessel both proximal and distal to the vessel lesion.[8]
There is some controversy of the accuracy of observing the lesion MLD itself, since many atherosclerotic lesions may create uneven "hills and valleys" within the lumen, making a true MLD difficult to obtain or estimate. Some research indicates calculating "area stenosis" is also a valid measure of actual vessel stenosis compared to diameter stenosis alone, but this requires additional analysis because a tracing of the lumen border must be performed. However, there are computer programs available to automatically perform this function. It may be helpful to obtain both percent diameter and area percent stenosis, especially since the two percentages may not always correlate with each other.[9]
An occlusion, or the blocking of all blood flow through a vessel, is considered 100% percent diameter stenosis.
Binary restenosis
Binary restenosis is traditionally defined as a reduction in the percent diameter stenosis of 50% or more (≥50%). It is also known as just "binary stenosis".[10] The term "binary" means that patients are placed in 2 groups, those who have ≥50% stenosis and those who have <50% stenosis. Binary restenosis is an epidemiological method of analyzing percent diameter stenosis for observing not only an individual patient, but also performing statistical techniques on group of patients to determine averages (descriptive measures of central tendency) or as a predictive variable.
Prevention
In the first stage of restenosis, administering anti-platelet drugs (called IIb/IIIa inhibitors) immediately after surgery greatly reduces the chance of a thrombosis occurring.
Drug-eluting stents, coated with pharmaceuticals that inhibit tissue growth and thus reduce the risk of restenosis from scar-tissue and cell proliferation, are now widely used.[11] These stents reduce the occurrence of restenosis, with clinical studies showing an incidence rate of 5% or lower.[3][12][13]
Treatment
If restenosis occurs without a stent, it is usually treated with more angioplasty. This treatment is also used if restenosis occurs at either the proximal or distal end of the stent.
If restenosis occurs within a stent (also known as in-stent stenosis), it may be treated with repeated angioplasty and insertion of another stent inside the original, often with a drug-eluting stent.[14]
Over the past 5 years, ISR is increasingly treated with a drug-coated balloon (DCB), which is a balloon coated with the same anticancer drugs that prevent restenosis, such as Paclitaxel.[15][16] The balloon avoids the need for a double layer of metal which is used when an in-stent restenosis is treated with another stent within the original stent. Additionally, DCB treatment does not leave an implant in the body and is designed for a faster drug delivery.
Alternative treatments include brachytherapy, or intracoronary radiation. The radiation kills cells and inhibits tissue growth (similar to a patient undergoing cancer therapy).[17]
Incidence
Rates of restenosis differ between devices (e.g., stent-grafts, balloon angioplasty, etc.) and location of procedure (i.e., centrally located in the heart, such as the coronary artery, or in peripheral vessels such as the popliteal artery in the leg, the pudendal artery in the pelvis, or the carotid artery in the neck).
Rates in cardiac procedures
In cardiac procedures, balloon angioplasty without stent implantation has been associated with a high incidence of restenosis, with rates ranging from 25% to 50%, and the majority of these patients need further angioplasty within 6 months.[18]
A 2010 study in India comparing coronary drug-eluting stents (DES) with coronary bare-metal stents (BMS) reported that restenosis developed in 23.1% of DES patients vs 48.8% in BMS patients, and female sex was found to be a statistically significant risk factor for developing restenosis.[19]
However, in newer-generation DES and BMS the restenosis rates are much lower. For example, the NORSTENT trial, presented in 2016, reports target-lesion revascularization rates of 5.3% and 10.3% for DES and BMS respectively.[13]
Rates in peripheral procedures
In peripheral procedures, rates are still high. A 2003 study of selective and systematic stenting for limb-threatening ischemia reported restenosis rates at 1 year follow-up in 32.3% of selective stenting patients and 34.7% of systematic stenting patients.[20]
The 2006 SIROCCO trial compared the sirolimus drug-eluting stent with a bare nitinol stent for atherosclerotic lesions of the subsartorial artery, reporting restenosis at 2 year follow-up was 22.9% and 21.1%, respectively.[21]
A 2009 study compared bare nitinol stents with percutaneous transluminal angioplasty (PTA) in subsartorial artery disease. At 1 year follow-up, restenosis was reported in 34.4% of stented patients versus 61.1% of PTA patients.[22]
See also
- Angioplasty
- Drug-eluting stent
- Neointimal hyperplasia
- Stent
- Images of restenosis with bare-metal stents and drug-eluting stents are here .
References
- Forgos, Richard N. (August 2004). "Restenosis After Angioplasty and Stenting".
- Bennett, M. R (2003). "In-Stent Stenosis: Pathology and Implications for the Development of Drug Eluting Stents". Heart. 89 (2): 218–24. doi:10.1136/heart.89.2.218. PMC 1767562. PMID 12527687.
- Hamid, H; Coltart, J (2007). "'Miracle stents' - a future without restenosis". McGill Journal of Medicine. 10 (2): 105–11. PMC 2323487. PMID 18523610.
- Kirchengast, M; Münter, K (1998). "Endothelin and restenosis". Cardiovascular Research. 39 (3): 550–5. doi:10.1016/S0008-6363(98)00143-6. PMID 9861296.
- Clowes, Alexander M.D. VascularWeb, Society for Vascular Surgery https://dev.vascularweb.org/research/Pages/prevention-of-neointimal-hyperplasia-taxol-,-rapamycin-,-and-radiation.aspx%5B%5D
- http://www.hkma.org/english/cme/clinicalcase/200703ans1.htm%5B%5D
- Kuntz, R. E.; Safian, R. D.; Carrozza, J. P.; Fishman, R. F.; Mansour, M.; Baim, D. S. (1992). "The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting". Circulation. 86 (6): 1827–35. doi:10.1161/01.CIR.86.6.1827. PMID 1451255.
- Meijboom, W. Bob; Van Mieghem, Carlos A.G.; Van Pelt, Niels; Weustink, Annick; Pugliese, Francesca; Mollet, Nico R.; Boersma, Eric; Regar, Eveline; et al. (2008). "Comprehensive Assessment of Coronary Artery Stenoses". Journal of the American College of Cardiology. 52 (8): 636–43. doi:10.1016/j.jacc.2008.05.024. PMID 18702967.
- Ota, H.; Takase, K.; Rikimaru, H.; Tsuboi, M.; Yamada, T.; Sato, A.; Higano, S.; Ishibashi, T.; Takahashi, S. (2005). "Quantitative Vascular Measurements in Arterial Occlusive Disease". Radiographics. 25 (5): 1141–58. doi:10.1148/rg.255055014. PMID 16160101.
- Foley 2004, p. 613-614. https://books.google.com/books?id=zz36NEQzk-YC&pg=PA613&lpg=PA613&dq=diameter+stenosis+was+calculated+by&source=bl&ots=TcvOqjEiRA&sig=JPUtoDuSPXqKHcx9vllai1dBSYg&hl=en&sa=X&ei=Y6-IT4cl5KaJAqfP7eIH&ved=0CDwQ6AEwATgU#v=onepage&q=diameter%20stenosis%20was%20calculated%20by&f=false%5B%5D
- Raffoul, Jad; Nasir, Ammar; Klein, Andrew J. P. (2018). "Technological Advances in Stent Therapies: a Year in Review". Current Treatment Options in Cardiovascular Medicine. 20 (5): 36. doi:10.1007/s11936-018-0630-2. ISSN 1092-8464. PMID 29627909.
- http://circinterventions.ahajournals.org/content/2/4/352.extract
- Fernández-Ruiz, Irene (2016). "Drug-eluting or bare-metal stents?". Nature Reviews Cardiology. 13 (11): 631. doi:10.1038/nrcardio.2016.160. ISSN 1759-5002. PMID 27629515.
- Jukema, J. Wouter; Ahmed, Tarek A. N.; Verschuren, Jeffrey J. W.; Quax, Paul H. A. (2012). "Restenosis after PCI. Part 2: prevention and therapy". Nature Reviews Cardiology. 9 (2): 79–90. doi:10.1038/nrcardio.2011.148. ISSN 1759-5002. PMID 21989052.
- Wu, Ridong; Li, Zilun; Wang, Mian; Chang, Guangqi; Yao, Chen; Wang, Shenming (June 2017). "Paclitaxel-coated versus uncoated balloon angioplasty for femoropopliteal artery in-stent restenosis". International Journal of Surgery. 42: 72–82. doi:10.1016/j.ijsu.2017.04.057.
- Kolachalama, Vijaya B.; Shazly, Tarek; Vipul C. Chitalia; Lyle, Chimera; Azar, Dara A.; Chang, Gary H. (2019-05-02). "Intrinsic coating morphology modulates acute drug transfer in drug-coated balloon therapy". Scientific Reports. 9 (1): 6839. doi:10.1038/s41598-019-43095-9. ISSN 2045-2322.
- Andras, Alina; Hansrani, Monica; Stewart, Marlene; Stansby, Gerard (2014-01-08). "Intravascular brachytherapy for peripheral vascular disease". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.cd003504.pub2. ISSN 1465-1858. PMC 6863108.
- Grech, E. D (2003). "Percutaneous coronary intervention. I: History and development". BMJ. 326 (7398): 1080–2. doi:10.1136/bmj.326.7398.1080. PMC 1125993. PMID 12750213.
- Mohan, S; Dhall, A (2010). "A comparative study of restenosis rates in bare metal and drug-eluting stents". The International Journal of Angiology. 19 (2): e66–72. doi:10.1055/s-0031-1278368. PMC 3005409. PMID 22477592.
- Becquemin, Jean-Pierre; Favre, Jean-Pierre; Marzelle, Jean; Nemoz, Chantal; Corsin, Caroline; Leizorovicz, Alain (2003). "Systematic versus selective stent placement after superficial femoral artery balloon angioplasty: A multicenter prospective randomized study". Journal of Vascular Surgery. 37 (3): 487–94. doi:10.1067/mva.2003.155. PMID 12618680.
- Duda, Stephan H.; Bosiers, Marc; Lammer, Johannes; Scheinert, Dierk; Zeller, Thomas; Oliva, Vincent; Tielbeek, Alexander; Anderson, John; et al. (2006). "Drug-Eluting and Bare Nitinol Stents for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery:Long-term Results from the SIROCCO Trial". Journal of Endovascular Therapy. 13 (6): 701–10. doi:10.1583/05-1704.1. PMID 17154704.
- Dick, Petra; Wallner, Hubert; Sabeti, Schila; Loewe, Christian; Mlekusch, Wolfgang; Lammer, Johannes; Koppensteiner, Renate; Minar, Erich; Schillinger, Martin (2009). "Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions". Catheterization and Cardiovascular Interventions. 74 (7): 1090–5. doi:10.1002/ccd.22128. PMID 19859954.