Rubidium azide
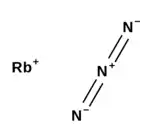
Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the azide ion (N–
3). Like most azides, it is explosive.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
rubidium(1+);azide | |
| Other names
Rubidium azide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| RbN3 | |
| Molar mass | 127.49 g·mol−1 |
| Appearance | Colorless needles[1] |
| Density | 2.79 g/cm3[1][2] |
| Melting point | 317–321 °C (603–610 °F; 590–594 K)[2][3] |
| Boiling point | Decomposes |
| 107.1 g/100 g (16°C) 114.1 g/100 g (17°C)[4] | |
| Solubility | 0.182 g/100 g (16°C, ethanol)[4] |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-0.1 kcal·mol−1[2] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Rubidium nitrate |
Other cations |
Lithium azide Sodium azide Potassium azide Silver azide Ammonium azide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Rubidium azide can be created by the reaction between rubidium sulfate and barium azide which results in formation of easily separated insoluble barium sulfate:[4]
In at least one study, rubidium azide was produced by the reaction between butyl nitrite, hydrazine monohydrate, and rubidium hydroxide:
This formula is typically used to synthesize potassium azide from caustic potash.[5]
Uses
Rubidium azide has been investigated for possible use in alkali vapor cells, which are components of atomic clocks, atomic magnetometers and atomic gyroscopes. Azides are desirable starting materials because they decompose into rubidium metal and nitrogen gas when exposed to UV light. According to one publication:
Among the different techniques used to fill microfabricated alkali vapor cell [sic], UV decomposition of rubidium azide (RbN3) into metallic Rb and nitrogen in Al2O3 coated cells is a very promising approach for low-cost wafer-level fabrication.[6]
Structure
At room temperature, rubidium azide has the same structure as potassium hydrogen fluoride; a distorted cesium chloride structure. At 315 °C and 1 atm, rubidium azide will transition to the normal cesium chloride structure. The II/I transition temperature of rubidium azide is within 2 °C of its melting point.[3]
Rubidium azide has a high pressure structure transition, which occurs at about 4.8 kilobars of pressure at 0 °C. The transition boundary of the II/III transition can be defined by the relationship , where is the pressure in kilobars and is the temperature in degrees Celsius.[3]
Reactions
As with all azides, it will decompose and release nitrogen gas when heated or severely shocked:
Hazards
At 4.1 kilobars of pressure and about 460 °C, rubidium azide will explosively decompose.[3] Under normal circumstances, it explodes at 395 °C.[2] It also decomposes upon exposure to ultraviolet light.[6]
Rubidium azide is very sensitive to mechanical shock, with an impact sensitivity comparable to that of TNT.[7]
Like all azides, rubidium azide is toxic.
References
- Perry, Dale (1995-05-17). Handbook of Inorganic Compounds. Online. p. 333. ISBN 9780849386718. Retrieved 31 January 2018.
- Hart, William; Beumel, O. F.; Whaley, Thomas (22 October 2013). The Chemistry of Lithium, Sodium, Potassium, Rubidium, Cesium and Francium: Pergamon Texts in Inorganic Chemistry. Online: Pergamon Press. p. 438. ISBN 9781483187570. Retrieved 31 January 2018.
- Pistorius, Carl W. F. T. (27 December 1968). "Phase Diagrams to High Pressures of the Univalent Azides Belonging to the Space Group D 4hI8-14/mcm" (PDF). Online. pp. 1, 4–5. Retrieved 1 February 2018.
- Hála, Jiri. "IUPAC-NIST Solubility Data Series. 79. Alkali and Alkaline Earth Metal Pseudohalides" (PDF). nist.gov. Retrieved 31 January 2018.
- Ogden, J. Steven; Dyke, John M.; Levason, William; Ferrante, Francesco; Gagliardi, Laura. "The Characterisation of Molecular Alkali-Metal Azides" (PDF). PMID 16491492. Retrieved 2 February 2018. Cite journal requires
|journal=(help) - Karlen, Sylvain; Gobet, Jean; Overstolz, Thomas; Haesler, Jacques; Lecomte, Steve (26 January 2017). "Lifetime assessment of RbN3-filled MEMS atomic vapor cells with Al2O3 coating" (PDF). Optics Express. 25 (3): 2187–2194. Bibcode:2017OExpr..25.2187K. doi:10.1364/OE.25.002187. PMID 29519066. Retrieved 17 March 2018.
- Babu, K. Ramesh; Vaitheeswaran, G. (2013). "Structure, elastic and dynamical properties of KN3 and RbN3: A van der Waals density functional study". Solid State Sciences. Advanced Centre of Research in High Energy Materials (ACRHEM), University of Hyderabad. 23: 17–25. arXiv:1311.0979. Bibcode:2013SSSci..23...17R. CiteSeerX 10.1.1.768.1309. doi:10.1016/j.solidstatesciences.2013.05.017.
| HN3 | He | ||||||||||||||||||
| LiN3 | Be(N3)2 | B(N3)3 | CH3N3, C(N3)4 |
N(N3)3,H2N—N3 | O | FN3 | Ne | ||||||||||||
| NaN3 | Mg(N3)2 | Al(N3)3 | Si(N3)4 | P | SO2(N3)2 | ClN3 | Ar | ||||||||||||
| KN3 | Ca(N3)2 | Sc(N3)3 | Ti(N3)4 | VO(N3)3 | Cr(N3)3, CrO2(N3)2 |
Mn(N3)2 | Fe(N3)2, Fe(N3)3 |
Co(N3)2, Co(N3)3 |
Ni(N3)2 | CuN3, Cu(N3)2 |
Zn(N3)2 | Ga(N3)3 | Ge | As | Se(N3)4 | BrN3 | Kr | ||
| RbN3 | Sr(N3)2 | Y | Zr(N3)4 | Nb | Mo | Tc | Ru(N3)63− | Rh(N3)63− | Pd(N3)2 | AgN3 | Cd(N3)2 | In | Sn | Sb | Te | IN3 | Xe(N3)2 | ||
| CsN3 | Ba(N3)2 | Hf | Ta | W | Re | Os | Ir(N3)63− | Pt(N3)62− | Au(N3)4− | Hg2(N3)2, Hg(N3)2 |
TlN3 | Pb(N3)2 | Bi(N3)3 |
Po | At | Rn | |||
| Fr | Ra(N3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(N3)3, Ce(N3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(N3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(N3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
