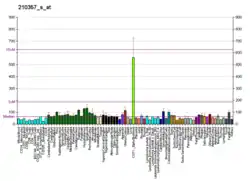
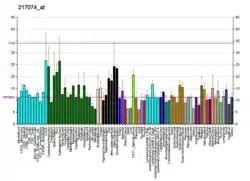
SMOX
Spermine oxidase is an enzyme that in humans is encoded by the SMOX gene.[5][6][7]
Function
The product of this gene is the polyamine oxidase. This enzyme potentially represents a new class of catabolic enzymes in the mammalian polyamine metabolic pathway capable of the efficient oxidation of polyamines. More than five transcript variants encoding four active isoenzymes have been identified for this gene, however, not all variants have been fully described. The characterized isoenzymes have distinctive biochemical characteristics and substrate specificities, suggesting the existence of additional levels of complexity in polyamine catabolism.[7]
References
- GRCh38: Ensembl release 89: ENSG00000088826 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027333 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA (July 2001). "Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure". Cancer Research. 61 (14): 5370–3. PMID 11454677.
- Murray-Stewart T, Wang Y, Devereux W, Casero RA (December 2002). "Cloning and characterization of multiple human polyamine oxidase splice variants that code for isoenzymes with different biochemical characteristics". The Biochemical Journal. 368 (Pt 3): 673–7. doi:10.1042/BJ20021587. PMC 1223052. PMID 12398765.
- "Entrez Gene: SMOX spermine oxidase".
Further reading
- Seiler N (June 2004). "Catabolism of polyamines". Amino Acids. 26 (3): 217–33. doi:10.1007/s00726-004-0070-z. PMID 15221502. S2CID 22119330.
- Hölttä E (January 1977). "Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase". Biochemistry. 16 (1): 91–100. doi:10.1021/bi00620a015. PMID 12798.
- Tsukada T, Furusako S, Maekawa S, Hibasami H, Nakashima K (1988). "Purification by affinity chromatography and characterization of porcine liver cytoplasmic polyamine oxidase". The International Journal of Biochemistry. 20 (7): 695–702. doi:10.1016/0020-711X(88)90164-4. PMID 3181599.
- Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW (November 2002). "Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin". The Biochemical Journal. 367 (Pt 3): 665–75. doi:10.1042/BJ20020720. PMC 1222929. PMID 12141946.
- Cervelli M, Polticelli F, Federico R, Mariottini P (February 2003). "Heterologous expression and characterization of mouse spermine oxidase". The Journal of Biological Chemistry. 278 (7): 5271–6. doi:10.1074/jbc.M207888200. PMID 12458219.
- Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA (May 2003). "Properties of purified recombinant human polyamine oxidase, PAOh1/SMO". Biochemical and Biophysical Research Communications. 304 (4): 605–11. doi:10.1016/S0006-291X(03)00636-3. PMID 12727196.
- Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, Federico R, Amendola R, Mariottini P (February 2004). "Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform". European Journal of Biochemistry. 271 (4): 760–70. doi:10.1111/j.1432-1033.2004.03979.x. PMID 14764092.
- Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA (December 2005). "Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines". The Journal of Biological Chemistry. 280 (48): 39843–51. doi:10.1074/jbc.M508177200. PMID 16207710.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.