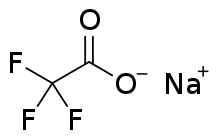
Sodium trifluoroacetate
Sodium trifluoroacetate is a chemical compound with a formula of CF3CO2Na. It is the sodium salt of trifluoroacetic acid. It is used as a source of trifluoromethylations.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Sodium trifluoroacetate | |
| Other names
Sodium perfluoroacetate Sodium 2,2,2-trifluoroacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.982 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2F3NaO2 | |
| Molar mass | 136.005 g·mol−1 |
| Appearance | White crystalline powder |
| Density | 1.49 g mL−1 |
| Melting point | 207 °C (405 °F; 480 K) |
| Boiling point | Decomposes |
| 625 g/L | |
| Solubility | soluble in alcohol, acetonitrile, dimethylformamide and most of polar organic solvents |
| Acidity (pKa) | 0.23 (conjugate acid) |
| Hazards | |
| Main hazards | Toxic, Irritant, Harmful to environment |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H300, H315, H319, H335, H400, H410 | |
| P261, P264, P270, P271, P273, P280, P301+310, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Non-flammable | |
| Related compounds | |
Other anions |
Sodium trichloroacetate |
Related compounds |
Sodium formate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Basicity
With the pKa of 0.23 for trifluoroacetic acid (compared to acetic acid, which has a pKa of 4.76), the trifluoroacetate ion is clearly a much weaker base. This is because of the electron-withdrawing effect on the 3 fluorine atoms adjacent to the carbon atom. With strong acids, such as hydrochloric acid or sulfuric acid, the trifluoroacetate ion can be protonated:
It also partially reacts with hydronium cations to form the acid:
The equilibrium is not complete because of the smaller pKa difference of the acid and hydronium.
Preparation
One convenient method is by dissolving an equivalent amount of sodium carbonate in 50% aqueous solution of trifluoroacetic acid. The solution is filtered and evaporated by vacuum evaporation (with special care to avoid decomposition of the salt by overheating). The solid obtained is dried under vacuum at 100 °C.[2]
Uses
Sodium trifluoroacetate is a useful reagent for trifluoromethylation.
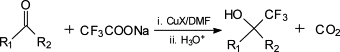 The trifluoromethylation process with sodium trifluoroacetate.
The trifluoromethylation process with sodium trifluoroacetate.
References
- "Trifluoromethylation of carbonyl compounds with sodium trifluoroacetate". Journal of Fluorine Chemistry. 126 (6): 937–940. June 2005. doi:10.1016/j.jfluchem.2005.04.012.
- Prakash, G. K. Surya; Mathew, Thomas (2010), "Sodium Trifluoroacetate", Encyclopedia of Reagents for Organic Synthesis, American Cancer Society, doi:10.1002/047084289x.rn01136, ISBN 9780470842898
