Strontium nitrate
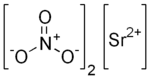
Strontium nitrate is an inorganic compound composed of the elements strontium, nitrogen and oxygen with the formula Sr(NO3)2. This colorless solid is used as a red colorant and oxidizer in pyrotechnics.
 | |
| Names | |
|---|---|
| IUPAC name
Strontium nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.107 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Sr(NO3)2 | |
| Molar mass | 211.630 g/mol (anhydrous) 283.69 g/mol (tetrahydrate) |
| Appearance | white crystalline solid |
| Density | 2.986 g/cm3 (anhydrous) 2.20 g/cm3 (tetrahydrate)[1] |
| Melting point | 570 °C (1,058 °F; 843 K) (anhydrous) 100 °C, decomposes (tetrahydrate) |
| Boiling point | 645 °C (1,193 °F; 918 K) decomposes |
| anhydrous: 710 g/L (18 °C) 660 g/L (20 °C) tetrahydrate: 604.3 g/L (0 °C) 2065 g/L (100 °C) | |
| Solubility | soluble in ammonia very slightly soluble in ethanol, acetone insoluble in nitric acid |
| −57.2·10−6 cm3/mol | |
| Structure | |
| cubic (anhydrous) monoclinic (tetrahydrate) | |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2750 mg/kg (rat, oral) |
| Related compounds | |
Other anions |
Strontium sulfate Strontium chloride |
Other cations |
Beryllium nitrate Magnesium nitrate Calcium nitrate Barium nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Strontium nitrate is typically generated by the reaction of nitric acid on strontium carbonate.[2]

Uses
Like many other strontium salts, strontium nitrate is used to produce a rich red flame in fireworks and road flares. The oxidizing properties of this salt are advantageous in such applications.[3]
Strontium nitrate can aid in eliminating and lessening skin irritations. When mixed with glycolic acid, strontium nitrate reduces the sensation of skin irritation significantly better than using glycolic acid alone.[4]
Biochemistry
As a divalent ion with an ionic radius similar to that of Ca2+ (1.13 Å and 0.99 Å respectively), Sr2+ ions resembles calcium's ability to traverse calcium-selective ion channels and trigger neurotransmitter release from nerve endings. It is thus used in electrophysiology experiments.
In popular culture
In his short story "A Germ-Destroyer", Rudyard Kipling refers to strontium nitrate as the main ingredient of the titular fumigant. Sr
References
- Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- Ward, R.; Osterheld, R. K.; Rosenstein, R. D. (1950). Strontium Sulfide and Selenide Phosphors. Inorganic Syntheses. 3. pp. 11–23. doi:10.1002/9780470132340.ch4. ISBN 978-0-470-13234-0.
- MacMillan, J. Paul; Park, Jai Won; Gerstenberg, Rolf; Wagner, Heinz; Köhler, Karl and Wallbrecht, Peter (2002) "Strontium and Strontium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_321
- Zhai H, Hannon W, Hahn GS, Pelosi A, Harper RA, Maibach HI (2000). "Strontium nitrate suppresses chemically-induced sensory irritation in humans". Contact Dermatitis. 42 (2): 98–100. doi:10.1034/j.1600-0536.2000.042002098.x. PMID 10703633.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 |
AgNO3 Ag(NO3)2 |
Cd(NO3)2 | In(NO3)3 | Sn | Sb(NO3)3 | Te | INO3 | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf(NO3)4 | Ta | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 |
Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
TlNO3 Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk | Cf | Es | Fm | Md | No | Lr | |||
