Succinaldehyde
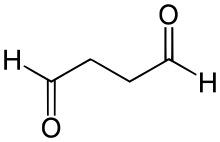
Succinaldehyde or succindialdehyde is an organic compound with the formula (CH2CHO)2. Typical of other dialdehydes, succinaldehyde is highly reactive. Usually, it is handled as the hydrates or methanol-derived acetal. It is a precursor to tropinone.[1] It is used as a crosslinking agent but is less widely used than the related dialdehyde glutaraldehyde.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butanedial | |
| Other names
Succinaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.304 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.09 |
| Appearance | colourless liquid |
| Density | 1.064 g/cm3 |
| Boiling point | 58 °C (136 °F; 331 K) at 9 mm Hg |
| with hydration | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
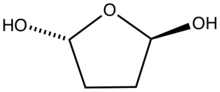
2,5-Dihydroxytetrahydrofuran, the hydrated form of succinaldehyde.
Succinaldehyde is generated by the oxidation of THF with chlorine followed by hydrolysis and by the hydroformylation of acrolein derivatives.
In aqueous solution, the molecule hydrates and cyclizes.[2] In methanol it converts to the cyclic acetal, 2,5-dimethoxyltetrahydrofuran.[3]
References
- U.S. Patent 2,710,883
- Hardy, P. M.; Nicholls, A. C.; Rydon, H. N. (1972). "The Hydration and Polymerisation of Succinaldehyde, Glutaraldehyde, and Adipaldehyde". Journal of the Chemical Society, Perkin Transactions 2 (15): 2270. doi:10.1039/P29720002270.
- Christian Kohlpaintner, Markus Schulte, Jürgen Falbe, Peter Lappe, Jürgen Weber (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.