Tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I.[1][2] Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone.[3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
8-Methyl-8-azabicyclo[3.2.1]octan-3-one | |
| Other names
3-Tropinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.756 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H13NO | |
| Molar mass | 139.195 g/mol |
| Appearance | Brown solid |
| Melting point | 42.5 °C (108.5 °F; 315.6 K) |
| Boiling point | (decomposes) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The first synthesis of tropinone was by Richard Willstätter in 1901. It started from the seemingly related cycloheptanone, but required many steps to introduce the nitrogen bridge; the overall yield for the synthesis path is only 0.75%.[4] Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine.[5]

Robinson's "double Mannich" reaction
The 1917 synthesis by Robinson is considered a classic in total synthesis[7] due to its simplicity and biomimetic approach. Tropinone is a bicyclic molecule, but the reactants used in its preparation are fairly simple: succinaldehyde, methylamine and acetonedicarboxylic acid (or even acetone). The synthesis is a good example of a biomimetic reaction or biogenetic-type synthesis because biosynthesis makes use of the same building blocks. It also demonstrates a tandem reaction in a one-pot synthesis. Furthermore, the yield of the synthesis was 17% and with subsequent improvements exceeded 90%.[4]
This reaction is described as an intramolecular "double Mannich reaction" for obvious reasons. It is not unique in this regard, as others have also attempted it in piperidine synthesis.[8][9]
In place of acetone, acetonedicarboxylic acid is known as the "synthetic equivalent" the 1,3-dicarboxylic acid groups are so-called "activating groups" to facilitate the ring forming reactions. The calcium salt is there as a "buffer" as it is claimed that higher yields are possible if the reaction is conducted at "physiological pH".
Reaction mechanism
The main features apparent from the reaction sequence below are:
- Nucleophilic addition of methylamine to succinaldehyde, followed by loss of water to create an imine
- Intramolecular addition of the imine to the second aldehyde unit and first ring closure
- Intermolecular Mannich reaction of the enolate of acetone dicarboxylate
- New enolate formation and new imine formation with loss of water for
- Second intramolecular Mannich reaction and second ring closure
- Loss of 2 carboxylic groups to tropinone
Some authors have actually tried to retain one of the CO2H groups.[10]
CO2R-tropinone has 4 stereoisomers, although the corresponding ecgonidine alkyl ester has only a pair of enantiomers.
Reduction of tropinone
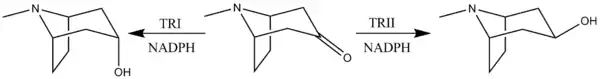
The reduction of tropinone is mediated by NADPH-dependent reductase enzymes, which have been characterized in multiple plant species.[11] These plant species all contain two types of the reductase enzymes, tropinone reductase I and tropinone reductase II. TRI produces tropine and TRII produces pseudotropine. Due to differing kinetic and pH/activity characteristics of the enzymes and by the 25-fold higher activity of TRI over TRII, the majority of the tropinone reduction is from TRI to form tropine.[12]
See also
- 2-Carbomethoxytropinone (2-CMT) an intermediate in the creation of ecgonine cocaine analogues
References
- Robinson, R. (1917). "LXIII. A Synthesis of Tropinone". Journal of the Chemical Society, Transactions. 111: 762–768. doi:10.1039/CT9171100762.
- Nicolaou, K. C.; Vourloumis, D.; Winssinger, N.; Baran, P. S. (2000). "The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century". Angewandte Chemie International Edition. 39 (1): 44–122. doi:10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L. PMID 10649349.
- Chemical Entities of Biological Interest Identification code: ChEBI:57851 "tropiniumone"
- Smit, Wim A.; Smit, William A.; Bochkov, Alekseĭ Feodosʹevich; Caple, Ron (1998). Organic Synthesis. doi:10.1039/9781847551573. ISBN 978-0-85404-544-0.
- Humphrey, A. J.; O'Hagan, D. (2001). "Tropane alkaloid biosynthesis. A century old problem unresolved". Natural Product Reports. Royal Society of Chemistry. 18 (5): 494–502. doi:10.1039/b001713m. PMID 11699882.
- Doble, Mukesh; Kruthiventi, Anil Kumar (2007). Green Chemistry and Engineering. Oxford: Elsevier. p. 34. ISBN 978-0-12-372532-5.
- Birch, A. J. (1993). "Investigating a Scientific Legend: The Tropinone Synthesis of Sir Robert Robinson, F.R.S". Notes and Records of the Royal Society of London. 47 (2): 277–296. doi:10.1098/rsnr.1993.0034. JSTOR 531792.
- Wang, S.; Sakamuri, S.; Enyedy, I. J.; Kozikowski, A. P.; Deschaux, O.; Bandyopadhyay, B. C.; Tella, S. R.; Zaman, W. A.; Johnson, K. M. (2000). "Discovery of a novel dopamine transporter inhibitor, 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketone, as a potential cocaine antagonist through 3D-database pharmacophore searching. Molecular modeling, structure-activity relationships, and behavioral pharmacological studies". Journal of Medicinal Chemistry. 43 (3): 351–360. doi:10.1021/jm990516x. PMID 10669562.
- Wang, S.; Sakamuri; Enyedy; Kozikowski; Zaman; Johnson (2001). "Molecular modeling, structure--activity relationships and functional antagonism studies of 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketones as a novel class of dopamine transporter inhibitors". Bioorganic & Medicinal Chemistry. 9 (7): 1753–1764. doi:10.1016/S0968-0896(01)00090-6. PMID 11425577.
- Findlay, S. P. (1957). "Concerning 2-Carbomethoxytropinone". Journal of Organic Chemistry. 22 (11): 1385–1394. doi:10.1021/jo01362a022.
- A. Portsteffen; B. Draeger & A. Nahrstedt (1992). "Two tropinone reducing enzymes from Datura stramonium transformed root cultures". Phytochemistry. 31 (4): 1135. doi:10.1016/0031-9422(92)80247-C.
- Boswell HD, Dräger B, McLauchlan WR, et al. (November 1999). "Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura". Phytochemistry. 52 (5): 871–8. doi:10.1016/S0031-9422(99)00293-9. PMID 10626376.


