VRLA battery
A valve regulated lead–acid (VRLA) battery, commonly known as a sealed lead–acid (SLA) battery,[1] is a type of lead–acid battery characterized by a limited amount of electrolyte ("starved" electrolyte) absorbed in a plate separator or formed into a gel; proportioning of the negative and positive plates so that oxygen recombination is facilitated within the cell; and the presence of a relief valve that retains the battery contents independent of the position of the cells.[2]

There are two primary types of VRLA batteries, absorbent glass mat (AGM) and gel cell.[3] Gel cells add silica dust to the electrolyte, forming a thick putty like gel. AGM (absorbent glass mat) batteries feature fiberglass mesh between the battery plates which serves to contain the electrolyte and separate the plates. Both types of VRLA batteries offer advantages and disadvantages compared to flooded vented lead–acid (VLA) batteries, as well as to each other.[4]
Due to their construction, the gel cell and AGM types of VRLA can be mounted in any orientation, and do not require constant maintenance. The term "maintenance free" is a misnomer as VRLA batteries still require cleaning and regular functional testing. They are widely used in large portable electrical devices, off grid power systems and similar roles, where large amounts of storage are needed at a lower cost than other low maintenance technologies like lithium ion.
Basic principle

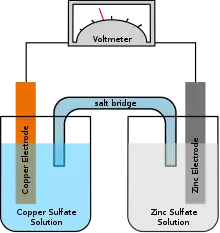
Lead–acid cells consist of two plates of lead, which serve as electrodes, suspended in an electrolyte consisting of diluted sulfuric acid. VRLA cells have the same chemistry. In AGM and gel type VRLA's, the electrolyte is immobilized. In AGM this is accomplished with a fiberglass mat; in gel batteries or "gel cells", the electrolyte is in the form of a paste like gel created by adding silica and other gelling agents to the electrolyte.[5]
When a cell discharges, the lead and diluted acid undergo a chemical reaction that produces lead sulfate and water. When a cell is subsequently charged, the lead sulfate and water are turned back into lead and acid. In all lead–acid battery designs, charging current must be adjusted to match the ability of the battery to absorb the energy. If the charging current is too great, electrolysis will occur, decomposing water into hydrogen and oxygen, in addition to the intended conversion of lead sulfate and water into lead dioxide, lead, and sulfuric acid (the reverse of the discharge process). If these gases are allowed to escape, as in a conventional flooded cell, the battery will need to have water (or electrolyte) added from time to time. In contrast, VRLA batteries retain generated gases within the battery as long as the pressure remains within safe levels. Under normal operating conditions the gases can then recombine within the battery itself, sometimes with the help of a catalyst, and no additional electrolyte is needed.[6][7] However, if the pressure exceeds safety limits, safety valves open to allow the excess gases to escape, and in doing so regulate the pressure back to safe levels (hence "valve regulated" in "VRLA").[8]
Construction
VRLA cells may be made of flat plates similar to a conventional flooded lead–acid battery, or may be made in a spiral roll form to make cylindrical cells.
VRLA batteries have a pressure relief valve which will activate when the battery starts building pressure of hydrogen gas, generally a result of being recharged.[8] Valve activation allows some of the gas or electrolyte to escape, thus decreasing the overall capacity of the battery. Rectangular cells may have valves set to operate as low as 1 or 2 PSI; round spiral cells, with metal external containers, can have valves set as high as 40 PSI.
The cell covers typically have gas diffusers built into them that allow safe dispersal of any excess hydrogen that may be formed during overcharge. They are not permanently sealed, but are designated to be maintenance free. They can be oriented in any manner, unlike normal lead–acid batteries, which must be kept upright to avoid acid spills and to keep the plates' orientation vertical. Cells may be operated with the plates horizontal (pancake style), which may improve cycle life.
At high overcharge currents, electrolysis of water occurs, expelling hydrogen and oxygen gas through the battery's valves. Care must be taken to prevent short circuits and rapid charging. Constant voltage charging is the usual, most efficient and fastest charging method for VRLA batteries, although other methods can be used.
VRLA batteries may be continually float charged at around 2.18-2.27 volts per cell at 25 °C, depending on the type and battery manufacturer specifications. An equalization charge cycle, having a higher voltage low current profile, may be occasionally utilized to partially reverse a battery sulfation condition; some SLA smart battery chargers either manually or automatically perform equalization cycles at infrequent times. Some designs can be fast charged (one hour) at high rates. Sustained charging at 2.7 V per cell will damage the cells. Constant current overcharging at high rates (rates faster than restoring the rated capacity in three hours) will exceed the capacity of the cell to recombine hydrogen and oxygen.
History
The first lead–acid gel battery was invented by Elektrotechnische Fabrik Sonneberg in 1934.[9] The modern gel or VRLA battery was invented by Otto Jache of Sonnenschein in 1957.[10] The first AGM cell was the Cyclon, patented by Gates Rubber Corporation in 1972 and now produced by EnerSys.[11] The cyclon is a spiral wound cell with thin lead foil electrodes. A number of manufacturers seized on the technology to implement it in cells with conventional flat plates. In the mid 1980’s, two UK companies, Chloride and Tungstone, simultaneously introduced ten year life AGM batteries in capacities up to 400 Ah, stimulated by a British Telecom specification for batteries for support of new digital exchanges. In the same period, Gates acquired another UK company, Varley, specialising in aircraft and military batteries. Varley adapted the Cyclon lead foil technology to produce flat plate batteries with exceptional high rate output. These gained approval for a variety of aircraft including the BAE 125 and 146 business jets, the Harrier and its derivative the AV8B, and some F16 variants as the first alternatives to then standard nickel cadmium (NiCd) batteries.[12]
Absorbent glass mat (AGM)
AGM batteries differ from flooded lead–acid batteries in that the electrolyte is held in the glass mats, as opposed to freely flooding the plates. Very thin glass fibers are woven into a mat to increase the surface area enough to hold a sufficient amount of electrolyte on the cells for their lifetime. The fibers that compose the fine glass mat do not absorb and are not affected by the acidic electrolyte. These mats are wrung out 2–5% after being soaked in acids just prior to finish manufacturing.
The plates in an AGM battery may be of any shape. Some are flat, whereas others are bent or rolled. Both deep cycle and starting type of AGM batteries, are built into a rectangular case according to Battery Council International (BCI) battery code specifications.
AGM batteries present better self discharging characteristics than conventional batteries within a wide range of temperatures.[13]
As with lead–acid batteries, in order to maximize the life of an AGM battery, it is important to follow the manufacturers charging specifications and the use of a voltage regulated charger is recommended.[14] There is a direct correlation between the depth of discharge (DOD) and the cycle life of the battery,[15] with differences between 500 and 1300 cycles depending on depth of discharge.
Gel battery

Originally a kind of gel cell was produced in the early 1930s for portable valve (tube) radio LT supply (2, 4 or 6V) by adding silica to the sulfuric acid.[16] By this time the glass case was being replaced by celluloid and later in 1930s other plastics. Earlier "wet" cells in glass jars used special valves to allow tilt from vertical to one horizontal direction in 1927 to 1931 or 1932.[17] The gel cells were less likely to leak when the portable set was handled roughly.
A modern gel battery (also known as a gel cell) is a VRLA battery with a gelified electrolyte; the sulfuric acid is mixed with fumed silica, which makes the resulting mass gel like and immobile. Unlike a flooded wet cell lead—acid battery, these batteries do not need to be kept upright. Gel batteries reduce the electrolyte evaporation, spillage (and subsequent corrosion problems) common to the wet cell battery, and boast greater resistance to shock and vibration. Chemically they are almost the same as wet (non sealed) batteries except that the antimony in the lead plates are replaced by calcium, and gas recombination can take place.
The modern gel formulation was invented by Otto Jache's and Heinz Schroeder's U.S. Patent 4,414,302 assigned to the German company Accumulatorenfabrik Sonnenschein. With gel electrolyte used as the separator, it was no longer such a critical and difficult to make component, as a result, the cycle life was increased, sometimes drastically, along with a reduction of the active material being shedded from the plates.
Gas recombination is used to make this type of battery without the requirement to occasionally add water to them in order to maintain the strength of the electrolyte, and are therefore referred to as maintenance free batteries. The one way valve on each cell is set at 2 psi, which allows for full recombination to take place within the sealed enclosure. When charging is complete and the battery is allowed to continue charging unregulated, oxygen is created by the overcharging condition on the positive plate. The oxygen then travels through the shrinkage cracks in the gel directly to the negative plate which is made from a high surface area pure sponge lead and causes a reaction that combines the oxygen with the hydrogen that is adsorbed on the surface of the sponge lead metal negative plate to create water that is retained in the cell. This chemical reaction removes the requirement to occasionally add water to the cells as there is no evaporation taking place from the sealed enclosure.
This sealed, non spill feature made it possible to make very small VRLA batteries (1–12 amp-hour range) that fit into the growing portable electronics market. A large market for inexpensive smaller sealed lead–acid batteries was generated quickly. Portable TV, light for news cameras, children's toy riding cars, emergency lighting, and UPS systems for computer backup, to name a few, were powered with small sealed VRLA batteries.
Applications
Many modern motorcycles and all-terrain vehicles (ATVs) on the market use AGM batteries to reduce likelihood of acid spilling during cornering, vibration, or after accidents, and for packaging reasons. The lighter, smaller battery can be installed at an odd angle if needed for the design of the motorcycle. Due to the higher manufacturing costs compared with flooded lead–acid batteries, AGM batteries are currently used on luxury vehicles. As vehicles become heavier and equipped with more electronic devices such as navigation and stability control, AGM batteries are being employed to lower vehicle weight and provide better electrical reliability compared with flooded lead–acid batteries.
5 series BMWs from March 2007 incorporate AGM batteries in conjunction with devices for recovering brake energy using regenerative braking and computer control to ensure the alternator charges the battery when the car is decelerating. Vehicles used in auto racing may use AGM batteries due to their vibration resistance.
Deep-cycle AGMs are also commonly used in off grid solar power and wind power installations as an energy storage bank and in large-scale amateur robotics, such as the FIRST and IGVC competitions.
AGM batteries are routinely chosen for remote sensors such as ice monitoring stations in the Arctic. AGM batteries, due to their lack of free electrolyte, will not crack and leak in these cold environments.
VRLA batteries are used extensively in power wheelchairs, as the extremely low gas and acid output makes them much safer for indoor use. VRLA batteries are also used in the uninterruptible power supply (UPS) as a back up when the electrical power goes off.
VRLA batteries are also the standard power source in sailplanes, due to their ability to withstand a variety of flight attitudes and a relatively large ambient temperature range with no adverse effects. However, charging regimes must be adapted with varying temperature.[18]
VRLA batteries are used in the US Nuclear Submarine fleet, due to their power density, elimination of gassing, reduced maintenance, and enhanced safety.[19]
AGM and gel-cell batteries are also used for recreational marine purposes, with AGM being more commonly available. AGM deep-cycle marine batteries are offered by a number of suppliers. They typically are favored for their low maintenance and spill-proof quality, although generally considered a less cost effective solution relative to traditional flooded cells.
In telecommunications applications, VRLA batteries that comply with criteria in Telcordia Technologies requirements document GR-4228, Valve-Regulated Lead-Acid (VRLA) Battery String Certification Levels Based on Requirements for Safety and Performance, are recommended for deployment in the Outside Plant (OSP) at locations such as Controlled Environmental Vaults (CEVs), Electronic Equipment Enclosures (EEEs), and huts, and in uncontrolled structures such as cabinets. Relative to VRLA in telecommunications, the use of VRLA Ohmic Measurement Type Equipment (OMTE) and OMTE-like measurement equipment is a fairly new process to evaluate telecommunications battery plants.[20] The proper use of ohmic test equipment allows battery testing without the need to remove batteries from service to perform costly and time-consuming discharge tests.
Comparison with flooded lead–acid cells
VRLA gel and AGM batteries offer several advantages compared with VRLA flooded lead–acid and conventional lead–acid batteries. The battery can be mounted in any position, since the valves only operate on over-pressure faults. Since the battery system is designed to be recombinant and eliminate the emission of gases on overcharge, room ventilation requirements are reduced, and no acid fume is emitted during normal operation. Flooded cell gas emissions are of little consequence in all but the smallest confined areas, and pose very little threat to a domestic user, so a wet cell battery designed for longevity gives lower costs per kWh. In a gel battery, the volume of free electrolyte that could be released on damage to the case or venting is very small. There is no need (or ability) to check the level of electrolyte or to top up water lost due to electrolysis, thus reducing inspection and maintenance requirements.[21] Wet-cell batteries can be maintained by a self-watering system or by topping up every three months. The requirement to add distilled water is normally caused by overcharging. A well-regulated system should not require top-up more often than every three months.
An underlying disadvantage with all lead–acid batteries is the requirement for a relatively long recharge cycle time arising from an inherent three-stage charging process: bulk charge, absorption charge, and (maintenance) float charge stages. All lead–acid batteries, irrespective of type, are quick to bulk charge to about 70% of capacity during which the battery will accept a large current input, determined at a voltage setpoint, within a few hours (with a charge source capable of supplying the design C-rate bulk stage current for a given Ah battery).
However, they then require a longer time spent in the current-tapering off intermediate absorption charge stage after the initial bulk charge, when the LA battery charge acceptance rate gradually reduces, and the battery will not accept a higher C-rate. When the absorption stage voltage setpoint is reached (and charge current has tapered off), the charger switches to a float voltage setpoint at a very low C-rate to maintain the battery's fully charged state indefinitely (the float stage offsets the battery's normal self-discharge over time).
If the charger fails to supply a sufficient absorption stage charge duration and C-rate (it 'plateaus' or times out, a common fault of cheap solar chargers), and a suitable float charge profile, the battery's capacity and longevity will be dramatically reduced.
To ensure maximum life, a lead–acid battery should be fully recharged as soon after a discharge cycle as possible to prevent sulfation, and kept at a full charge level by a float source when stored or idle (or stored dry new from the factory, an uncommon practice today).
When working a discharge cycle, a LA battery should be kept at a depth of discharge (DOD) of less than 50%, ideally no more than 20-40% DOD; a true[22] LA deep-cycle battery can be taken to a lower DOD (even an occasional 80%), but these greater DOD cycles always impose a longevity price.
Lead–acid battery lifetime cycles will vary with the care given, with best care they may achieve 500 to 1000 cycles. With less careful use, a lifetime as few as 100 cycles might be expected (all dependent upon the use environment too).
Because of calcium added to its plates to reduce water loss, a sealed AGM or gel battery recharges more quickly than a flooded lead–acid battery of either VRLA or conventional design.[23][24] Compared to flooded batteries, VRLA batteries are more vulnerable to thermal runaway during abusive charging. The electrolyte cannot be tested by hydrometer to diagnose improper charging that can reduce battery life.[24]
AGM automobile batteries are typically about twice the price of flooded-cell batteries in a given Battery Council International (BCI) size group; gel batteries as much as five times the price.
AGM and gel VRLA batteries:
- Have shorter recharge time than flooded lead–acid batteries.[25]
- Cannot tolerate overcharging: overcharging leads to premature failure.[25]
- Have shorter useful life, compared to properly maintained wet-cell battery.[25]
- Discharge significantly less hydrogen gas.[25]
- AGM batteries are by nature, safer for the environment, and safer to use.
- Can be used or positioned in any orientation.
References
- Eismin, Thomas K. (2013). Aircraft Electricity and Electronics (Sixth ed.). McGraw Hill Professional. p. 48. ISBN 007179915X.
- David B. Linden, Thomas Reddy, Handbook of Batteries Third Edition, McGraw-Hill, 2002, ISBN ISBN 0-07-135978-8, chapter 24 "Valve Regulated Lead Acid Batteries"
- "Exploding Lead Acid Batteries, Mines Safety Bulletin No. 150". Australia: Queensland Government. 2015-10-27. Retrieved 2020-02-17.
- "Selecting the Proper Lead-Acid Technology" (PDF). Trojan Battery Company, California, USA. 2018. Retrieved 2020-02-17.
- Wagner, R (2004-03-09). "13.3 Gel batteries". In Moseley, Patrick T; et al. (eds.). Valve-Regulated Lead–Acid Batteries. p. 446. ISBN 9780444507464.
- Robert Nelson, "The Basic Chemistry of Gas Recombination in Lead–Acid Batteries", JOM 53 (1) (2001)
- "The Basic Chemistry of Gas Recombination in Lead–Acid Batteries". TMS.org.
- Ronald Dell, David Anthony James Rand, Robert Bailey, Jr., Understanding Batteries,Royal Society of Chemistry, 2001, ISBN 0854046054 p. 101, pp.120-122
- "A Brief History of Batteries and Stored Energy" (PDF). Netaworld.org. Retrieved 19 February 2019.
- "Handbook for Gel-VRLA-Batteries : Part 1 : Basic Principles, Design, Features" (PDF). Sonnenschein.org. Retrieved 19 February 2019.
- John Devitt (1997). "An account of the development of the first valve-regulated lead/acid cell". Journal of Power Sources. 64 (1–2): 153–156. Bibcode:1997JPS....64..153D. doi:10.1016/S0378-7753(96)02516-5.
- Kevin Desmond, "Jache, Otto", Innovators in Battery Technology: Profiles of 95 Influential Electrochemists, McFarland, 2016 ISBN 1476622787.
- "Technical Manual: Powersports Batteries" (PDF). YuasaBatteries.com. GS Yuasa. Archived from the original (PDF) on 2017-07-12. Retrieved 2019-12-25.
- "AGM Charging : Technical Support Desk". Support.rollsbattery.com. Retrieved 19 February 2019.
- "AGM Discharge Characteristics : Modified on: Mon, 6 Oct, 2014". Support.rollsbattery.com. Retrieved 19 February 2019.
- Watterson, Michael (2014-06-28). "Exide Gel-Cel Accumulator JSK2 Power-S Chloride Electrical". RadioMuseum.org. Retrieved 2015-03-01.
- Walchhofer, Hans Martin & Watterson, Michael (2013-11-27). "Super Range Portable (without tuning dial) Radio McMichael L". RadioMuseum.org. Retrieved 2015-03-01.
- Linden, Reddy (ed), Handbook of batteries, third ed, 2002
- "Exide Earns First-Ever Production Contract Awarded by U.S. Navy for Valve-Regulated Submarine Batteries; Shift to Advanced Product Prompts Closure of Kankakee, Illinois, Battery Plant". Business Wire. 2005. Retrieved 7 September 2016.
- GR-3169-CORE, Generic Requirements for Valve-Regulated Lead-Acid (VRLA) Battery Ohmic Measurement Type Equipment (OMTE).
- Fink, Donald G.; Beaty, H. Wayne (1978). Standard Handbook for Electrical Engineers (Eleventh ed.). New York: McGraw-Hill. pp. 11–116. ISBN 0-07-020974-X.
- Collins, Rod (April 7, 2015). "What is a Deep Cycle Battery?".
- Barre, Harold (1997). Managing 12 Volts: How to Upgrade, Operate and Troubleshoot 12 Volt Electrical Systems. Summer Breeze Publishing. p. 44. ISBN 978-0-9647386-1-4.(stating sealed battery plates are hardened with calcium to reduce water loss which "raises the batteries' internal resistance and prevents rapid charging.")
- Sterling, Charles (2009). "FAQ: What is the Best Battery System to Use for an Auxiliary Charging System". Archived from the original on 16 March 2012. Retrieved 2 February 2012.
- Calder, Nigel (1996). Boatowner's Mechanical and Electrical Manual (2nd ed.). pp. 11. ISBN 978-0-07-009618-9.
Further reading
Books and papers
- Valve-Regulated Lead-Acid Batteries. Edited by Patrick T. Moseley, Jurgen Garche, C.D. Parker, D.A.J. Rand. p202
- Vinal, G.W. (1955 Jan 01) Storage batteries. A general treatise on the physics and chemistry of secondary batteries and their engineering applications. Energy Citations Database (ECD) : Document #7308501
- John McGavack. The Absorption of Sulfur Dioxide by the Gel of Silicic Acid. Eschenbach Print. Company, 1920.
Patents
- U.S. Patent 417,392 Treatment Of Porous Pots For Electric Batteries. Erhard Ludwig Mayer and Henry Liepmann
- U.S. Patent 3,271,199 Solid Acid Storage Battery Electrolyte. Alexander Koenig et al.
- U.S. Patent 4,134,192 Composite battery plate grid
- U.S. Patent 4,238,557 Lead acid battery plate with starch coated glass fibers