4-Aminophenylmercuric acetate
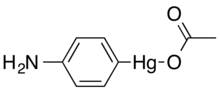
4-Aminophenylmercuric acetate (CH3CO2HgC6H4NH2, also known as 4-(Acetoxymercurio)aniline or APMA), is an organomercurial compound and thiol-blocking reagent used in experimental biology and chemistry to activate matrix metalloproteinases and collagenase proteolytic enzymes.[1][2] The material is highly toxic.
 | |
| Names | |
|---|---|
| IUPAC name
Acetyloxy-(4-aminophenyl)mercury | |
| Identifiers | |
3D model (JSmol) |
|
| 3664749 | |
| ChemSpider | |
| ECHA InfoCard | 100.025.907 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H9HgNO2 | |
| Molar mass | 351.757 g·mol−1 |
| Appearance | off-white powder |
| 5 mM | |
| Solubility | DMSO |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H300, H310, H330, H373, H400, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
APMA has a molecular weight of 351.8 g/mol and appears as a white powder with a slight yellowish cast. Its melting temperature is 163–165 °C.[3] APMA is soluble in water to concentrations as high as 5 mM, and in DMSO to concentrations of 10 M or more. In 100% acetic acid, an APMA solution of 50 mg/mL is a light translucent yellow.[3]
Protein modification
APMA is known to activate matrix metalloproteinase enzymes and collagenase.[4] APMA activates proteolytic enzymes by reacting with cysteines at the amino terminal domains that bind zinc, near the location of the enzyme active site.[4]
Toxicity
APMA and APMA vapors are highly toxic or fatal in contact with skin, or if inhaled or swallowed.[3]
See also
- 4-Chloromercuribenzoic acid - an organomercury compound that is used as a protease inhibitor
- Phenylmercury acetate - a structurally related organomercury compound historically used as a preservative in paints
References
- Rosenfeldt, Mathias (2005). "The organomercurial 4-aminophenylmercuric acetate, independent of matrix metalloproteinases, induces dose-dependent activation/ inhibition of platelet aggregation". Thromb Haemost. 93 (2): 326. doi:10.1160/th04-08-0541. PMID 15711750.
- Sellers, Anthony; Cartwright, Elizabeth; Murphy, Gillian; Reynolds, John (1977). "Evidence that Latent Collagenases are Enzyme-Inhibitor Complexes". Biochemical Journal. 163: 303–307. doi:10.1042/bj1630303. PMC 1164697. PMID 194584.
- "p-Aminotophenylmercuric acetate material safety data sheet" (PDF). sigmaaldrich.com. Sigma Aldrich. Retrieved 7 June 2018.
- Lundblad, Roger (2014). Chemical reagents for protein modification (Fourth ed.). CRC Press. p. 234. Retrieved 7 June 2018.
| AcOH | He | |||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | |||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | |||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | |||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | |||
| CsOAc | Ba(OAc)2 | * | Lu(OAc)3 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | ||
| Fr | Ra | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||
| * | La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | ||||||
| ** | Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | ||||||