CECXG
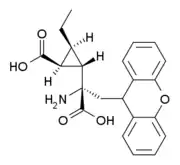
CECXG (3'-ethyl-LY-341,495) is a research drug which acts as a potent and selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with reasonable selectivity for mGluR3. While it is some five times less potent than LY-341,495 at mGluR3, it has 38x higher affinity for mGluR3 over mGluR2,[1] making it one of the few ligands available that is able to distinguish between these two closely related receptor subtypes.[2][3][4]
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H23NO5 |
| Molar mass | 381.428 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Collado I, Ezquerra J, Mazón A, Pedregal C, Yruretagoyena B, Kingston AE, Tomlinson R, Wright RA, Johnson BG, Schoepp DD (October 1998). "2,3'-disubstituted-2-(2'-carboxycyclopropyl)glycines as potent and selective antagonists of metabotropic glutamate receptors". Bioorganic & Medicinal Chemistry Letters. 8 (20): 2849–54. doi:10.1016/S0960-894X(98)00510-1. PMID 9873635.
- Jan Egebjerg, Povl Krogsgaard-Larsen, Arne Schousboe. Glutamate and gaba receptors and transporters: structure, function and pharmacology. pp 171-173. Taylor & Francis, 2002. ISBN 0-7484-0881-9
- Sørensen, U. (2003). "Synthesis and Structure–Activity Relationship Studies of Novel 2-Diarylethyl Substituted (2-Carboxycycloprop-1-yl)glycines as High-Affinity Group II Metabotropic Glutamate Receptor Ligands". Bioorganic & Medicinal Chemistry. 11 (2): 197–205. doi:10.1016/S0968-0896(02)00387-5.
- Ure, J.; Baudry, M.; Perassolo, M. (2006). "Metabotropic glutamate receptors and epilepsy". Journal of the Neurological Sciences. 247 (1): 1–9. doi:10.1016/j.jns.2006.03.018. PMID 16697014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.