Cadmium bromide
Cadmium bromide is a cream-coloured crystalline ionic cadmium salt of hydrobromic acid that is soluble in water. It is very toxic, along with other cadmium compounds.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cadmium(II) bromide | |
| Other names
Cadmium dibromide | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.241 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CdBr2 | |
| Molar mass | 272.22 g/mol |
| Appearance | white to pale yellow crystalline solid |
| Density | 5.192 g/cm3, solid |
| Melting point | 568 °C (1,054 °F; 841 K) |
| Boiling point | 844 °C (1,551 °F; 1,117 K) |
| 56.3 g/100 mL (0 °C) 98.8 g/100 mL (20 °C) 160 g/100 mL (100 °C) | |
| Solubility | soluble in alcohol, ether, acetone and liquid ammonia. |
| -87.3·10−6 cm3/mol | |
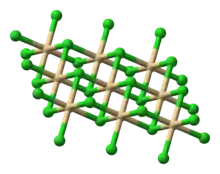
| Structure | |
| Rhombohedral, hr9, SpaceGroup = R-3m, No. 166 | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H302, H312, H332, H400, H410 | |
| P220, P273, P280, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
225 mg/kg, oral (rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1027] TWA 0.005 mg/m3 (as Cd)[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
Ca [9 mg/m3 (as Cd)][1] |
| Related compounds | |
Other anions |
Cadmium chloride, Cadmium iodide |
Other cations |
Zinc bromide, Calcium bromide, Magnesium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
It is used in the manufacturing of photographic film, engraving and lithography.
Preparation
Cadmium bromide is prepared by heating cadmium with bromine vapor. Also the compound can be prepared by the treatment of dry cadmium acetate with glacial acetic acid and acetyl bromide. Alternatively, it can be obtained by dissolving cadmium or cadmium oxide in hydrobromic acid and evaporating the solution to dryness under helium in an inert atmosphere.[2]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- Patnaik, P. (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 978-0-07-049439-8.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
