Hafnium tetrabromide
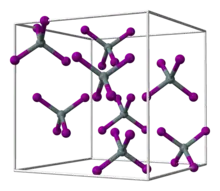
Hafnium tetrabromide is the inorganic compound with the formula HfBr4. It is the most common bromide of hafnium. It is a colorless, diamagnetic moisture sensitive solid that sublimes in vacuum.[1] It adopts a structure very similar to that of zirconium tetrabromide, featuring tetrahedral Hf centers, in contrast to the polymeric nature of hafnium tetrachloride.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.001 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Br4Hf | |
| Appearance | colorless solid |
| Density | 5.094 g/cm3 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- W. Thomas, H. Elias "Darstellung von HfCl4 und HfBr4 durch Umsetzung von Hafnium mit Geschmolzenen Metallhalogeniden" Journal of Inorganic and Nuclear Chemistry 1976, Volume 38, Pages 2227–2229. doi:10.1016/0022-1902(76)80199-6
- Berdonosov, S. S.; Berdonosova, D. G.; Lapitskii, A. V.; Vlasov, L. G. "X-ray study of hafnium tetrabromide" Zhurnal Neorganicheskoi Khimii, 1963, vol. 8, 531-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.