Echinoderm
An echinoderm /ɪˈkaɪnoʊdɜːrm/ is any member of the phylum Echinodermata /ɪˌkaɪnoʊˈdɜːrmətə/ (from Ancient Greek ἐχῖνος echīnos "hedgehog" and δέρμα derma "skin")[2] of marine animals. The adults are recognizable by their (usually five-point) radial symmetry, and include starfish, sea urchins, sand dollars, and sea cucumbers, as well as the sea lilies or "stone lilies".[3] Adult echinoderms are found on the sea bed at every ocean depth, from the intertidal zone to the abyssal zone. The phylum contains about 7000 living species,[4] making it the second-largest grouping of deuterostomes (a superphylum), after the chordates (which include the vertebrates, such as birds, fishes, mammals, and reptiles). Echinoderms are the largest phylum that has no freshwater or terrestrial members.
| Echinoderms | |
|---|---|
 | |
| Various echinoderms | |
| Scientific classification | |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| Superphylum: | Deuterostomia |
| Clade: | Ambulacraria |
| Phylum: | Echinodermata Bruguière, 1791 [ex Klein, 1734] |
| Subphyla and classes[1] | |
|
Homalozoa † Gill & Caster, 1960
†=Extinct | |
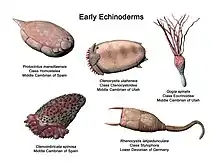
Aside from the hard-to-classify Arkarua (a Precambrian animal with echinoderm-like pentamerous radial symmetry), the first definitive members of the phylum appeared near the start of the Cambrian. One group of Cambrian echinoderms, the cinctans (Homalozoa), which are close to the base of the echinoderm origin, have been found to possess external gills used for filter feeding, similar to those possessed by chordates and hemichordates.[5]
The echinoderms are important both ecologically and geologically. Ecologically, there are few other groupings so abundant in the biotic desert of the deep sea, as well as shallower oceans. Most echinoderms are able to reproduce asexually and regenerate tissue, organs, and limbs; in some cases, they can undergo complete regeneration from a single limb. Geologically, the value of echinoderms is in their ossified skeletons, which are major contributors to many limestone formations, and can provide valuable clues as to the geological environment. They were the most used species in regenerative research in the 19th and 20th centuries. Further, some scientists hold that the radiation of echinoderms was responsible for the Mesozoic Marine Revolution.
Taxonomy and evolution
Along with the chordates and hemichordates, echinoderms are deuterostomes, one of the two major divisions of the bilaterians, the other being the protostomes. During the early development of the embryo, in deuterostomes, the blastopore (the first opening to form) becomes the anus whereas in the protostomes, it becomes the mouth. In deuterostomes, the mouth develops at a later stage, at the opposite end of the blastula from the blastopore, and a gut forms connecting the two.[6] The larvae of echinoderms have bilateral symmetry but this is lost during metamorphosis when their bodies are reorganised and develop the characteristic radial symmetry of the echinoderm, typically pentamerism.[7] The characteristics of adult echinoderms are the possession of a water vascular system with external tube feet and a calcareous endoskeleton consisting of ossicles connected by a mesh of collagen fibres.[8]
Phylogeny
Early analyses gave inconsistent results, the main hypotheses being that the Ophiuroidea were sister to the Asteroidea, or that they were sister to the (Holothuroidea + Echinoidea).[9] However, a 2014 analysis of 219 genes from all classes of echinoderms gave the following phylogenetic tree.[10] An independent analysis in 2015 of RNA transcriptomes from 23 species across all classes of echinoderms gave the same tree.[9]
- External phylogeny
| Bilateria |
| ||||||||||||||||||||||||||||||
- Internal phylogeny
| Echino‑ |
| ||||||||||||||||||||||||
| dermata |

Diversity
There are a total of about 7,000 extant species of echinoderm as well as about 13,000 extinct species.[8] They are found in habitats ranging from shallow intertidal areas to abyssal depths. Two main subdivisions are traditionally recognised: the more familiar motile Eleutherozoa, which encompasses the Asteroidea (starfish, 1,745 recent species), Ophiuroidea (brittle stars, 2,300 species), Echinoidea (sea urchins and sand dollars, 900 species) and Holothuroidea (sea cucumbers, 1,430 species); and the Pelmatozoa, some of which are sessile while others move around. These consist of the Crinoidea (feather stars and sea lilies, 580 species) and the extinct blastoids and Paracrinoids.[11] A fifth class of Eleutherozoa consisting of just three species, the Concentricycloidea (sea daisies), were recently merged into the Asteroidea.[12] The fossil record includes a large number of other classes which do not appear to fall into any extant crown group.

Fossil history
The oldest known echinoderm fossil may be Arkarua from the Precambrian of Australia. It is a disc-like fossil with radial ridges on the rim and a five-pointed central depression marked with radial lines. However, no stereom or internal structure showing a water vascular system is present and the identification is inconclusive.[14]
The first universally accepted echinoderms appear in the Lower Cambrian period, asterozoans appeared in the Ordovician and the crinoids were a dominant group in the Paleozoic.[13] Echinoderms left behind an extensive fossil record.[13] It is hypothesised that the ancestor of all echinoderms was a simple, motile, bilaterally symmetrical animal with a mouth, gut and anus. This ancestral stock adopted an attached mode of life and suspension feeding, and developed radial symmetry as this was more advantageous for such an existence. The larvae of all echinoderms are even now bilaterally symmetrical and all develop radial symmetry at metamorphosis. The starfish and crinoids still attach themselves to the seabed while changing to their adult form.[15]
The first echinoderms later gave rise to free-moving groups. The evolution of endoskeletal plates with stereom structure and of external ciliary grooves for feeding were early echinoderm developments.[16] The Paleozoic echinoderms were globular, attached to the substrate and were orientated with their oral surfaces upwards. The fossil echinoderms had ambulacral grooves extending down the side of the body, fringed on either side by brachioles, structures very similar to the pinnules of a modern crinoid. It seems probable that the mouth-upward orientation is the primitive state and that at some stage, all the classes of echinoderms except the crinoids reversed this to become mouth-downward. Before this happened, the podia probably had a feeding function as they do in the crinoids today. Their locomotor function came later, after the re-orientation of the mouth when the podia were in contact with the substrate for the first time.[15]
Anatomy and physiology
Echinoderms evolved from animals with bilateral symmetry. Although adult echinoderms possess pentaradial, or five-sided, symmetry, echinoderm larvae are ciliated, free-swimming organisms that organize in bilateral symmetry which makes them look like embryonic chordates. Later, the left side of the body grows at the expense of the right side, which is eventually absorbed. The left side then grows in a pentaradially symmetric fashion, in which the body is arranged in five parts around a central axis.[17] Within the Asterozoa, there can be a few exceptions from the rule. The starfish genus Leptasterias normally have six arms, although five-armed individuals can occur. Also the Brisingida have six armed species. Amongst the brittle stars, six-armed species such as Ophiothela danae, Ophiactis savignyi, and Ophionotus hexactis exists, and Ophiacantha vivipara often has more than six.[18]
Echinoderms exhibit secondary radial symmetry in portions of their body at some stage of life. This, however, is an adaptation to their sessile existence. They developed from other members of the Bilateria and exhibit bilateral symmetry in their larval stage. Many crinoids and some seastars exhibit symmetry in multiples of the basic five, with starfish such as Labidiaster annulatus known to possess up to fifty arms, and the sea-lily Comaster schlegelii having two hundred.[19]
Skin and skeleton





Echinoderms have a mesodermal skeleton composed of calcareous plates or ossicles. Each one of these, even the articulating spine of a sea urchin, is composed mineralogically of a crystal of calcite. If solid, these would form a heavy skeleton, so they have a sponge-like porous structure known as stereom.[20] Ossicles may be fused together, as in the test of sea urchins, or may articulate with each other as in the arms of sea stars, brittle stars and crinoids. The ossicles may be flat plates or bear external projections in the form of spines, granules or warts and they are supported by a tough epidermis (skin). Skeletal elements are also deployed in some specialized ways, such as the "Aristotle's lantern" mouthparts of sea urchins used for grinding, the supportive stalks of crinoids and the structural "lime ring" of sea cucumbers.[17]
Despite the robustness of the individual skeletal modules complete skeletons of starfish, brittle stars and crinoids are rare in the fossil record. This is because they quickly disarticulate (disconnect from each other) once the encompassing skin rots away, and in the absence of tissue there is nothing to hold the plates together. The modular construction is a result of the growth system employed by echinoderms, which adds new segments at the centre of the radial limbs, pushing the existing plates outwards and lengthening the arms. Sea urchins on the other hand are often well preserved in chalk beds or limestone. During fossilization, the cavities in the stereom are filled in with calcite that is in crystalline continuity with the surrounding material. On fracturing such rock, distinctive cleavage patterns can be seen and sometimes even the intricate internal and external structure of the test.[21]
The epidermis consists of cells responsible for the support and maintenance of the skeleton, as well as pigment cells, mechanoreceptor cells (which detect motion on the animal's surface), and sometimes gland cells which secrete sticky fluids or even toxins. The varied and often vivid colours of echinoderms are produced by the action of skin pigment cells. These are produced by a variable combination of coloured pigments, such as the dark melanin, red carotinoids, and carotene proteins, which can be blue, green, or violet. These may be light-sensitive, and as a result many echinoderms change appearance completely as night falls. The reaction can happen quickly – the sea urchin Centrostephanus longispinus changes from jet black to grey-brown in just fifty minutes when exposed to light.[22]
One characteristic of most echinoderms is a special kind of tissue known as catch connective tissue. This collagenous material can change its mechanical properties in a few seconds or minutes through nervous control rather than by muscular means. This tissue enables a starfish to change from moving flexibly around the seabed to becoming rigid while prying open a bivalve mollusc or preventing itself from being extracted from a crevice. Similarly, sea urchins can lock their normally mobile spines rigidly as a defensive mechanism when attacked.[23]
The water vascular system
Echinoderms possess a unique water vascular system. This is a network of fluid-filled canals derived from the coelom (body cavity) that function in gas exchange, feeding, sensory reception and locomotion. This system varies between different classes of echinoderm but typically opens to the exterior through a sieve-like madreporite on the aboral (upper) surface of the animal. The madreporite is linked to a slender duct, the stone canal, which extends to a ring canal that encircles the mouth or oesophagus. From this, radial canals extend along the arms of asteroids and adjoin the test in the ambulacral areas of echinoids. Short lateral canals branch off the radial canals, each one ending in an ampulla. Part of the ampulla can protrude through a pore (or a pair of pores in sea urchins) to the exterior and is known as a podium or tube feet. The water vascular system assists with the distribution of nutrients throughout the animal's body and is most obviously expressed in the tube feet which can be extended or contracted by the redistribution of fluid between the foot and the internal sac.[24]
The organization of the system is somewhat different in ophiuroids where the madreporite may be on the oral surface and the podia lack suckers.[25] In holothuroids, the podia may be reduced or absent and the madreporite opens into the body cavity so that the circulating liquid is coelomic fluid rather than sea water.[26] The arrangements in crinoids is similar to asteroids but the tube feet lack suckers and are used to pass food particles captured by the arms towards the central mouth. In the asteroids, the same wafting motion is employed to move the animal across the ground.[27] Sea urchins use their feet to prevent the larvae of encrusting organisms from settling on their surfaces; potential settlers are moved to the urchin's mouth and eaten. Some burrowing sea stars extend their elongated dorsal tube feet to the surface of the sand or mud above and use them to absorb oxygen from the water column.[28]
Other organs
Echinoderms possess a simple digestive system which varies according to the animal's diet. Starfish are mostly carnivorous and have a mouth, oesophagus, two-part stomach, intestine and rectum, with the anus located in the centre of the aboral body surface. With a few exceptions, the members of the order Paxillosida do not possess an anus.[29][30] In many species of starfish, the large cardiac stomach can be everted and digest food outside the body. In other species, whole food items such as molluscs may be ingested.[31] Brittle stars have a blind gut with no intestine or anus. They have varying diets and expel food waste through their mouth.[32] Sea urchins are herbivores and use their specialised mouthparts to graze, tear and chew algae and sometimes other animal or vegetable material. They have an oesophagus, a large stomach and a rectum with the anus at the apex of the test.[33] Sea cucumbers are mostly detritivores, sorting through the sediment with their buccal tentacles which are modified tube feet. Sand and mud accompanies their food through their simple gut which has a long coiled intestine and a capacious cloaca.[34] Crinoids are passive suspension feeders, catching plankton with their outstretched arms. Boluses of mucus-trapped food are passed to the mouth which is linked to the anus by a loop consisting of a short oesophagus and longer intestine.[35]
The coelomic cavities of echinoderms are complex. Aside from the water vascular system, echinoderms have a haemal coelom (or haemal system, the "haemal" being a misnomer), a perivisceral coelom, a gonadal coelom and often also a perihaemal coelom (or perihaemal system).[36] During development, echinoderm coelom is divided in metacoel, mesocoel and protocoel (also called somatocoel, hydrocoel and axocoel, respectively).[37] The water vascular system, haemal system and perihaemal system form the tubular coelomic system.[38] Echinoderms are an exception having both a coelomic circulatory system (i.e., the water vascular system) and a haemal circulatory system (i.e., the haemal and perihaemal systems).[39]
Haemal and perihaemal systems are derived from the coelom and form an open and reduced circulatory system. This usually consists of a central ring and five radial vessels. There is no true heart and the blood often lacks any respiratory pigment. Gaseous exchange occurs via dermal branchiae or papulae in starfish, genital bursae in brittle stars, peristominal gills in sea urchins and cloacal trees in sea cucumbers. Exchange of gases also takes place through the tube feet. Echinoderms lack specialized excretory (waste disposal) organs and so nitrogenous waste, chiefly in the form of ammonia, diffuses out through the respiratory surfaces.[24]
The coelomic fluid contains the coelomocytes, or immune cells. There are several types of immune cells, which vary among classes and species. All classes possess a type of phagocytic amebocyte, which engulf invading particles and infected cells, aggregate or clot, and may be involved in cytotoxicity. These cells are usually larger and granular, and are suggested to be a main line of defense against potential pathogens.[40] Depending on the class, echinoderms may have spherule cells (for cytotoxicity, inflammation, and anti-bacterial activity), vibratile cells (for coelomic fluid movement and clotting), and crystal cells (potential osmoregulatory cells in sea cucumbers),.[40][41] The coelomocytes also secrete Anti-Microbial Peptides (AMPs) against bacteria, and have a set of lectins and complement proteins as part of an innate immune system that is still being characterized.[2]
Echinoderms have a simple radial nervous system that consists of a modified nerve net consisting of interconnecting neurons with no central brain, although some do possess ganglia. Nerves radiate from central rings around the mouth into each arm or along the body wall; the branches of these nerves coordinate the movements of the organism and the synchronisation of the tube feet. Starfish have sensory cells in the epithelium and have simple eyespots and touch-sensitive tentacle-like tube feet at the tips of their arms. Sea urchins have no particular sense organs but do have statocysts that assist in gravitational orientation, and they have sensory cells in their epidermis, particularly in the tube feet, spines and pedicellariae. Brittle stars, crinoids and sea cucumbers in general do not have sensory organs but some burrowing sea cucumbers of the order Apodida have a single statocyst adjoining each radial nerve and some have an eyespot at the base of each tentacle.[42]
The gonads occupy much of the body cavities of sea urchins and sea cucumbers, while the less voluminous crinoids, brittle stars and starfish have two gonads in each arm. While the ancestral condition is considered to be the possession of one genital aperture, many organisms have multiple gonopores through which eggs or sperm may be released.[42]
Regeneration

Many echinoderms have remarkable powers of regeneration. Many species routinely autotomize and regenerate arms and viscera. Sea cucumbers often discharge parts of their internal organs if they perceive themselves to be threatened. The discharged organs and tissues are regenerated over the course of several months. Sea urchins are constantly replacing spines lost through damage. Sea stars and sea lilies readily lose and regenerate their arms. In most cases, a single severed arm cannot grow into a new starfish in the absence of at least part of the disc.[43][44][45][46] However, in a few species a single arm can survive and develop into a complete individual[44][45][46] and in some species, the arms are intentionally detached for the purpose of asexual reproduction. During periods when they have lost their digestive tracts, sea cucumbers live off stored nutrients and absorb dissolved organic matter directly from the water.[47]
The regeneration of lost parts involves both epimorphosis and morphallaxis. In epimorphosis stem cells—either from a reserve pool or those produced by dedifferentiation—form a blastema and generate new tissues. Morphallactic regeneration involves the movement and remodelling of existing tissues to replace lost parts. Direct transdifferentiation of one type of tissue to another during tissue replacement is also observed.[48]
The robust larval growth is responsible for many echinoderms being used as popular model organisms in developmental biology.[49]
Reproduction
Sexual reproduction
Echinoderms become sexually mature after approximately two to three years, depending on the species and the environmental conditions. They are nearly all gonochoric, though a few species are hermaphroditic. The eggs and sperm cells are typically released into open water, where fertilization takes place. The release of sperm and eggs is synchronised in some species, usually with regard to the lunar cycle. In other species, individuals may aggregate during the reproductive season, thereby increasing the likelihood of successful fertilisation. Internal fertilisation has currently been observed in three species of sea star, three brittle stars and a deep water sea cucumber. Even at abyssal depths, where no light penetrates, synchronisation of reproductive activity in echinoderms is surprisingly frequent.[50]
Some echinoderms brood their eggs. This is especially common in cold water species where planktonic larvae might not be able to find sufficient food. These retained eggs are usually few in number and are supplied with large yolks to nourish the developing embryos. In starfish, the female may carry the eggs in special pouches, under her arms, under her arched body or even in her cardiac stomach.[51] Many brittle stars are hermaphrodites. Egg brooding is quite common and usually takes place in special chambers on their oral surfaces, but sometimes the ovary or coelom is used.[52] In these starfish and brittle stars, direct development without passing through a bilateral larval stage usually takes place.[53] A few sea urchins and one species of sand dollar carry their eggs in cavities, or near their anus, holding them in place with their spines.[54] Some sea cucumbers use their buccal tentacles to transfer their eggs to their underside or back where they are retained. In a very small number of species, the eggs are retained in the coelom where they develop viviparously, later emerging through ruptures in the body wall.[55] In some species of crinoid, the embryos develop in special breeding bags, where the eggs are held until sperm released by a male happens to find them.[56]
Asexual reproduction
One species of seastar, Ophidiaster granifer, reproduces asexually by parthenogenesis.[57] In certain other asterozoans, the adults reproduce asexually for a while before they mature after which time they reproduce sexually. In most of these species, asexual reproduction is by transverse fission with the disc splitting in two. Regrowth of both the lost disc area and the missing arms occur[46][58] so that an individual may have arms of varying lengths. Though in most species at least part of the disc is needed for complete regeneration, in a few species of sea stars, a single severed arm can grow into a complete individual over a period of several months.[44][45][46] In at least some of these species, they actively use this as a method of asexual reproduction.[44][59] A fracture develops on the lower surface of the arm and the arm pulls itself free from the body which holds onto the substrate during the process.[59] During the period of regrowth, they have a few tiny arms and one large arm, thus often being referred to as "comets".[45][59]
Asexual reproduction by transverse fission has also been observed in adult sea cucumbers. Holothuria parvula uses this method frequently, an individual splitting into two a little in front of the midpoint. The two-halves each regenerate their missing organs over a period of several months but the missing genital organs are often very slow to develop.[60]
The larvae of some echinoderm species are capable of asexual reproduction. This has long been known to occur among starfish and brittle stars but has been more recently observed in a sea cucumber, a sand dollar and a sea urchin. These species belong to four of the major classes of echinoderms except crinozoans (as of 2011).[61] Asexual reproduction in the planktonic larvae occurs through numerous modes. They may autotomise parts that develop into secondary larvae, grow buds or undergo paratomy. The parts that are autotomised or the buds may develop directly into fully formed larvae or may develop through a gastrula or even a blastula stage. The parts that develop into the new larvae vary from the preoral hood (a mound like structure above the mouth), the side body wall, the postero-lateral arms or their rear ends.[61][62][63]
The process of cloning is a cost borne by the larva both in resources as well as in development time. Larvae have been observed to undergo this process when food is plentiful[64] or temperature conditions are optimal.[63] It has also been suggested that cloning may occur to make use of the tissues that are normally lost during metamorphosis.[65] Recent research has shown that the larvae of some sand dollars clone themselves when they detect predators (by sensing dissolved fish mucus).[63][65] Asexual reproduction produces many smaller larvae that escape better from planktivorous fish.[66]
Larval development
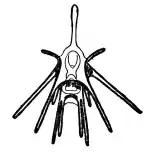
The development of an echinoderm begins with a bilaterally symmetrical embryo, with a coeloblastula developing first. Gastrulation marks the opening of the "second mouth" that places echinoderms within the deuterostomes, and the mesoderm, which will host the skeleton, migrates inwards. The secondary body cavity, the coelom, forms by the partitioning of three body cavities. The larvae are mostly planktonic but in some species the eggs are retained inside the female and in some the female broods the larvae.[67]
The larvae pass through several stages, which have specific names derived from the taxonomic names of the adults or from their appearance. For example, a sea urchin has an 'echinopluteus' larva while a brittle star has an 'ophiopluteus' larva. A starfish has a 'bipinnaria' larva, which develops into a multi-armed 'brachiolaria' larva. A sea cucumber's larva is an 'auricularia' while a crinoid's is a 'vitellaria'. All these larvae are bilaterally symmetrical and have bands of cilia with which they swim; some, usually known as 'pluteus' larvae, have arms. When fully developed they settle on the seabed to undergo metamorphosis, and the larval arms and gut degenerate. The left hand side of the larva develops into the oral surface of the juvenile, while the right side becomes the aboral surface. At this stage the bilateral symmetry is lost and radial symmetry develops.[67][68]
The planktotrophic larva is considered to be the ancestral larval type for echinoderms but after 500 million years of larval evolution, about 68% of species whose development is known have a lecithotrophic larval type.[11] The provision of a yolk-sac means that smaller numbers of eggs are produced, the larvae have a shorter development period, smaller dispersal potential but a greater chance of survival. There seems to be an evolutionary trend towards a "lower-risk–lower-gain" strategy of direct development.[11]
Distribution and habitat
Echinoderms are globally distributed in almost all depths, latitudes and environments in the ocean. They reach highest diversity in reef environments but are also widespread on shallow shores, around the poles – refugia where crinoids are at their most abundant – and throughout the deep ocean, where bottom-dwelling and burrowing sea cucumbers are common – sometimes accounting for up to 90% of organisms. While almost all echinoderms are benthic – that is, they live on the sea floor – some sea-lilies can swim at great velocity for brief periods of time, and a few deep-sea sea cucumbers are fully floating. Some crinoids are pseudo-planktonic, attaching themselves to floating logs and debris, although this behaviour was exercised most extensively in the Paleozoic, before competition from such organisms as barnacles restricted the extent of the behaviour.[69]
The larvae of echinoderms, especially starfish and sea urchins, are pelagic, and with the aid of ocean currents can be transported for great distances, reinforcing the global distribution of the phylum.[70]
Mode of life
Locomotion

Echinoderms primarily use their tube feet to move about, though some sea urchins also use their spines. The tube feet typically have a tip shaped like a suction pad in which a vacuum can be created by contraction of muscles. This along with some stickiness provided by the secretion of mucus provides adhesion. Waves of tube feet contractions and relaxations move along the adherent surface and the animal moves slowly along.[71]
Brittle stars are the most agile of the echinoderms, raising their discs and taking strides when moving. The two forward arms grip the substrate with their tube feet, the two side arms "row", the hindermost arm trails and the animal moves in jerks. The arm spines provide traction and when moving among objects, the supple arms can coil around things. A few species creep around on pointed tube feet.[71] Starfish extend their tube feet in the intended direction of travel and grip the substrate by suction, after which the feet are drawn backwards. The movement of multiple tube feet, coordinated in waves, moves the animal forward, but progress is slow.[72] Some burrowing starfish have points rather than suckers on their tube feet and they are able to "glide" across the seabed at a faster rate.[73]
Sea urchins use their tube feet to move around in a similar way to starfish. Some also use their articulated spines to push or lever themselves along or lift their oral surfaces off the substrate. If a sea urchin is overturned, it can extend its tube feet in one ambulacral area far enough to bring them within reach of the substrate and then successively attach feet from the adjoining area until it is righted. Some species bore into rock and they usually do this by grinding away at the surface with their mouthparts.[74]
Sea cucumbers are generally sluggish animals. Many can move on the surface or burrow through sand or mud using peristaltic movements and some have short tube feet on their under surface with which they can creep along in the manner of a starfish. Some species drag themselves along by means of their buccal tentacles while others can expand and contract their body or rhythmically flex it and "swim". Many live in cracks, hollows and burrows and hardly move at all. Some deep water species are pelagic and can float in the water with webbed papillae forming sails or fins.[75]
The majority of crinoids are motile but the sea lilies are sessile and attached to hard substrates by stalks. These stems can bend and the arms can roll and unroll and that is about the limit of the sea lily's movement, although a few species can relocate themselves on the seabed by crawling. The sea feathers are unattached and usually live in crevices, under corals or inside sponges with their arms the only visible part. Some sea feathers emerge at night and perch themselves on nearby eminences to better exploit the food-bearing current. Many species can "walk" across the seabed, raising their body with the help of their arms. Many can also swim with their arms but most are largely sedentary, seldom moving far from their chosen place of concealment.[76]
Feeding
The modes of feeding vary greatly between the different echinoderm taxa. Crinoids and some brittle stars tend to be passive filter-feeders, enmeshing suspended particles from passing water; most sea urchins are grazers, sea cucumbers deposit feeders and the majority of starfish are active hunters.
Crinoids are suspension feeders and spread their arms wide to catch particles floating past. These are caught by the tube feet on the pinnules, moved into the ambulacral grooves, wrapped in mucus and conveyed to the mouth by the cilia lining the grooves.[77] The exact dietary requirements of crinoids have been little researched but in the laboratory they can be fed with diatoms.[78]
Basket stars are suspension feeders, raising their branched arms to collect zooplankton, while brittle stars use several methods of feeding, though usually one predominates. Some are suspension feeders, securing food particles with mucus strands, spines or tube feet on their raised arms. Others are scavengers and feeders on detritus. Others again are voracious carnivores and able to lasso their waterborne prey with a sudden encirclement by their flexible arms. The limbs then bend under the disc to transfer the food to the jaws and mouth.[79]
Many sea urchins feed on algae, often scraping off the thin layer of algae covering the surfaces of rocks with their specialised mouthparts known as Aristotle's lantern. Other species devour smaller organisms, which they may catch with their tube feet. They may also feed on dead fish and other animal matter.[80] Sand dollars may perform suspension feeding and feed on phytoplankton, detritus, algal pieces and the bacterial layer surrounding grains of sand.[81]
Many sea cucumbers are mobile deposit or suspension feeders, using their buccal podia to actively capture food and then stuffing the particles individually into their buccal cavities. Others ingest large quantities of sediment, absorb the organic matter and pass the indigestible mineral particles through their guts. In this way they disturb and process large volumes of substrate, often leaving characteristic ridges of sediment on the seabed. Some sea cucumbers live infaunally in burrows, anterior-end down and anus on the surface, swallowing sediment and passing it through their gut. Other burrowers live anterior-end up and wait for detritus to fall into the entrances of the burrows or rake in debris from the surface nearby with their buccal podia.[82]
Nearly all starfish are detritivores or carnivores, though a few are suspension feeders. Small fish landing on the upper surface may be captured by pedicilaria and dead animal matter may be scavenged but the main prey items are living invertebrates, mostly bivalve molluscs. To feed on one of these, the starfish moves over it, attaches its tube feet and exerts pressure on the valves by arching its back. When a small gap between the valves is formed, the starfish inserts part of its stomach into the prey, excretes digestive enzymes and slowly liquefies the soft body parts. As the adductor muscle of the shellfish relaxes, more stomach is inserted and when digestion is complete, the stomach is returned to its usual position in the starfish with its now liquefied bivalve meal inside it. The same everted stomach process is used by other starfish to feed on sponges, sea anemones, corals, detritus and algal films.[83]
Defense mechanisms
.jpg.webp)
Despite their low nutrition value and the abundance of indigestible calcite, echinoderms are the prey of many organisms, such as crabs, sharks, sea birds and other echinoderms. Antipredator defences include the presence of spines, toxins, which can be inherent or delivered through the tube feet, and the discharge of sticky entangling threads by sea cucumbers. Although most echinoderm spines are blunt, those of the crown-of-thorns starfish are long and sharp and can cause a painful puncture wound as the epithelium covering them contains a toxin.[84] Because of their catch connective tissue, which can change rapidly from a flaccid to a rigid state, echinoderms are very difficult to dislodge from crevices. Certain sea cucumbers have a cluster of cuvierian tubules which can be ejected as long sticky threads from their anus and entangle and permanently disable an attacker. Another defensive strategy sometimes adopted by sea cucumbers is to rupture the body wall and discharge the gut and internal organs. The animal has a great regenerative capacity and will regrow the lost parts later.[85] Starfish and brittle stars may undergo autotomy when attacked, an arm becoming detached which may distract the predator for long enough for the animal to escape. Some starfish species can "swim" away from what may be danger, foregoing the regrowth by not losing limbs.[86] It is not unusual to find starfish with arms of different sizes in various stages of regrowth.[87]
Ecology
Echinoderms are numerous and relatively large invertebrates and play an important role in marine, benthic ecosystems.[11] The grazing of sea urchins reduces the rate of colonization of bare rock by settling organisms but also keeps algae in check, thereby enhancing the biodiversity of coral reefs. The burrowing of sand dollars, sea cucumbers and some starfish stirs up the sediment and depletes the sea floor of nutrients. Their digging activities increases the depth to which oxygen can seep and allows a more complex ecological tier-system to develop. Starfish and brittle stars prevent the growth of algal mats on coral reefs, which might otherwise obstruct the filter-feeding constituent organisms.[88] Some sea urchins can bore into solid rock and this bioerosion can destabilise rock faces and release nutrients into the ocean. Coral reefs are also bored into in this way but the rate of accretion of carbonate material is often greater than the erosion produced by the sea urchin.[89] It has been estimated that echinoderms capture and sequester about 0.1 gigatonnes of carbon per year as calcium carbonate, making them important contributors in the global carbon cycle.[90]
Echinoderms sometimes have large population swings which can cause marked consequences for ecosystems. An example is the change from a coral-dominated reef system to an alga-dominated one that resulted from the mass mortality of the tropical sea urchin Diadema antillarum in the Caribbean in 1983.[91] Sea urchins are among the main herbivores on reefs and there is usually a fine balance between the urchins and the kelp and other algae on which they graze. A diminution of the numbers of predators (otters, lobsters and fish) can result in an increase in urchin numbers causing overgrazing of kelp forests with the result that an alga-denuded "urchin barren" forms.[92] On the Great Barrier Reef, an unexplained increase in the numbers of crown-of-thorns starfish (Acanthaster planci), which graze on living coral tissue, has had considerable impact on coral mortality and coral reef biodiversity.[93]
Echinoderms form part of the diet of many organisms such as bony fish, sharks, eider ducks, gulls, crabs, gastropod molluscs, sea otters, Arctic foxes and humans. Larger starfish prey on smaller ones and the great quantity of eggs and larvae produced form part of the zooplankton, consumed by many marine creatures. Crinoids are relatively free from predation.[88] The body cavities of many sea cucumbers and some starfish provide a habitat for parasitic or symbiotic organisms including fish, crabs, worms and snails.[94]
Use by humans
As food and medicine


In 2010, 373,000 tonnes of echinoderms were harvested, mainly for consumption, but also in traditional Chinese medicine.[95] These were mainly sea cucumbers (158,000 tonnes) and sea urchins (73,000 tonnes).[96] Sea cucumbers are considered a delicacy in some countries of south east Asia; as such, they are in imminent danger of being over-harvested.[97] Popular species include the pineapple roller Thelenota ananas (susuhan) and the red Holothuria edulis. These and other species are colloquially known as bêche de mer or trepang in China and Indonesia. The sea cucumbers are boiled for twenty minutes and then dried both naturally and later over a fire which gives them a smoky tang. In China they are used as a basis for gelatinous soups and stews.[98] Both male and female gonads of sea urchins are also consumed particularly in Japan, Peru, Spain and France. The taste is described as soft and melting, like a mixture of seafood and fruit. The quality is assessed by the colour which can range from light yellow to bright orange.[99] At the present time, some trials of breeding sea urchins in order to try to compensate the overexploitation of this resource have been made.[100]
In research
Sea urchins are used in research, particularly as model organisms in developmental biology[101] and ecotoxicology.[102][103] Strongylocentrotus purpuratus and Arbacia punctulata are used for this purpose in embryological studies.[104] The large size and the transparency of the eggs enables the observation of sperm cells in the process of fertilising ova.[101] The arm regeneration potential of brittle stars is being studied in connection with understanding and treating neurodegenerative diseases in humans.[105]
Other uses
The calcareous tests or shells of echinoderms are used as a source of lime by farmers in areas where limestone is unavailable and some are used in the manufacture of fish meal.[106] Four thousand tons of the animals are used annually for these purposes. This trade is often carried out in conjunction with shellfish farmers, for whom the starfish pose a major threat by eating their cultured stock. Other uses for the starfish they recover include the manufacture of animal feed, composting and drying for the arts and craft trade.[105]
See also
- List of prehistoric echinoderm genera – Wikipedia list article
- Echinopedinidae
References
- Stöhr, Sabine (2014). "Echinodermata". WoRMS. World Register of Marine Species. Retrieved 23 February 2014.
- "echinoderm". Online Etymology Dictionary.
- "Sea Lily". Science Encyclopedia. Retrieved 5 September 2014.
- "Animal Diversity Web - Echinodermata". University of Michigan Museum of Zoology. Retrieved 26 August 2012.
- "Computer simulations reveal feeding in early animal".
- Dorit, Walker & Barnes 1991, pp. 777–779.
- Richard Fox. "Asterias forbesi". Invertebrate Anatomy OnLine. Lander University. Retrieved 19 May 2012.
- Wray, Gregory A. (1999). "Echinodermata: Spiny-skinned animals: sea urchins, starfish, and their allies". Tree of Life. Retrieved 19 October 2012.
- Escriva, Hector; Reich, Adrian; Dunn, Casey; Akasaka, Koji; Wessel, Gary (2015). "Phylogenomic Analyses of Echinodermata Support the Sister Groups of Asterozoa and Echinozoa". PLOS ONE. 10 (3): e0119627. Bibcode:2015PLoSO..1019627R. doi:10.1371/journal.pone.0119627. ISSN 1932-6203. PMC 4368666. PMID 25794146.
- Telford, M. J.; Lowe, C. J.; Cameron, C. B.; Ortega-Martinez, O.; Aronowicz, J.; Oliveri, P.; Copley, R. R. (2014). "Phylogenomic analysis of echinoderm class relationships supports Asterozoa". Proceedings of the Royal Society B: Biological Sciences. 281 (1786): 20140479. doi:10.1098/rspb.2014.0479. PMC 4046411. PMID 24850925.
- Uthicke, Sven; Schaffelke, Britta; Byrne, Maria (1 January 2009). "A boom–bust phylum? Ecological and evolutionary consequences of density variations in echinoderms". Ecological Monographs. 79: 3–24. doi:10.1890/07-2136.1.
- Siera104. "Echinodermata". Retrieved 15 March 2008.
- Waggoner, Ben (16 January 1995). "Echinodermata: Fossil Record". Introduction to the Echinodermata. Museum of Paleontology: University of California at Berkeley. Retrieved 14 March 2013.
- Smith, Dave (28 September 2005). "Vendian Animals: Arkarua". Retrieved 14 March 2013.
- Dorit, Walker & Barnes 1991, pp. 792–793.
- UCMP Berkeley. "Echinodermata: Morphology". University of California Museum of Paleontology. Retrieved 21 March 2011.
- Ruppert, Fox & Barnes 2004, p. 873.
- Australian Echinoderms: Biology, Ecology and Evolution
- Messing, Charles. "Crown and calyx". Charles Messing's Crinoid Pages. Archived from the original on 29 October 2013. Retrieved 29 July 2012.
- Behrens, Peter; Bäuerlein, Edmund (2007). Handbook of Biomineralization: Biomimetic and bioinspired chemistry'. Wiley-VCH. p. 393. ISBN 978-3-527-31805-6.
- Davies, A. Morley (1925). An Introduction to Palaeontology. Thomas Murby. pp. 240–241.
- Weber, W.; Dambach, M. (April 1974). "Light-sensitivity of isolated pigment cells of the sea urchin Centrostephanus longispinus". Cell and Tissue Research. 148 (3): 437–440. doi:10.1007/BF00224270. PMID 4831958. S2CID 1669387.
- Motokawa, Tatsuo (May 1984). "Connective tissue catch in echinoderms". Biological Reviews. 59 (2): 255–270. doi:10.1111/j.1469-185X.1984.tb00409.x. S2CID 86391676.
- Dorit, Walker & Barnes 1991, pp. 780–791.
- Dorit, Walker & Barnes 1991, pp. 784–785.
- Dorit, Walker & Barnes 1991, pp. 788–789.
- Dorit, Walker & Barnes 1991, pp. 780–783.
- Ruppert, Fox & Barnes 2004, p. 905.
- Echinoderm Nutrition
- Rigby, P. Robin; Iken, Katrin; Shirayama, Yoshihisa (2007). Sampling Biodiversity in Coastal Communities: NaGISA Protocols for Seagrass and Macroalgal Habitats. NUS Press. p. 44. ISBN 978-9971693688.
- Ruppert, Fox & Barnes 2004, p. 885.
- Ruppert, Fox & Barnes 2004, p. 891.
- Ruppert, Fox & Barnes 2004, pp. 902–904.
- Ruppert, Fox & Barnes 2004, p. 912.
- Ruppert, Fox & Barnes 2004, p. 920.
- J. Moore. An Introduction to the Invertebrates, Cambridge Univ. Press, 2nd ed., 2006, p. 245.
- C. Hickman Jr., L. Roberts, A. Larson. Animal Diversity. Granite Hill Publishers, 2003. p. 271.
- "Macrobenthos of the North Sea - Echinodermata > Introduction". etibioinformatics.nl.
- Nielsen, Claus. Animal Evolution: Interrelationships of the Living Phyla. 3rd ed. Oxford, UK: Oxford University Press, 2012, p. 78
- Ramirez-Gomez, Fransisco (27 September 2010). "Echinoderm Immunity". Invertebrate Survival Journal.
- Smith, Courtney (January 2010). "Echinoderm Immunity". Advances in Experimental Medicine and Biology. 708: 260–301. doi:10.1007/978-1-4419-8059-5_14. ISBN 978-1-4419-8058-8. PMID 21528703.
- Ruppert, Fox & Barnes 2004, pp. 872–929.
- Edmondson, C.H (1935). "Autotomy and regeneration of Hawaiian starfishes" (PDF). Bishop Museum Occasional Papers. 11 (8): 3–20.
- McAlary, Florence A (1993). Population Structure and Reproduction of the Fissiparous Seastar, Linckia columbiae Gray, on Santa Catalina Island, California. 3rd California Islands Symposium. National Park Service. Retrieved 15 April 2012.
- See last paragraph in review above AnalysisHotchkiss, Frederick H. C. (1 June 2000). "On the Number of Rays in Starfish". American Zoologist. 40 (3): 340–354. doi:10.1093/icb/40.3.340. Retrieved 14 July 2011.
- Fisher, W. K. (1 March 1925). "Asexual Reproduction in the Starfish, Sclerasterias" (PDF). Biological Bulletin. 48 (3): 171–175. doi:10.2307/1536659. ISSN 0006-3185. JSTOR 1536659. Retrieved 15 July 2011.
- Dobson, W. E.; S. E. Stancyk; L. A. Clements; R. M. Showman (1 February 1991). "Nutrient Translocation during Early Disc Regeneration in the Brittlestar Microphiopholis gracillima (Stimpson) (Echinodermata: Ophiuroidea)". Biol Bull. 180 (1): 167–184. doi:10.2307/1542439. JSTOR 1542439. PMID 29303627. Retrieved 14 July 2011.
- Mashanov, Vladimir S.; Igor Yu. Dolmatov; Thomas Heinzeller (1 December 2005). "Transdifferentiation in Holothurian Gut Regeneration". Biol Bull. 209 (3): 184–193. doi:10.2307/3593108. JSTOR 3593108. PMID 16382166. S2CID 27847466. Retrieved 15 July 2011.
- Hart, M. W. (January–February 2002). "Life history evolution and comparative developmental biology of echinoderms". Evolution and Development. 4 (1): 62–71. doi:10.1046/j.1525-142x.2002.01052.x. PMID 11868659. S2CID 39175905.
- Young, Craig M.; Eckelbarger, Kevin J. (1994). Reproduction, Larval Biology, and Recruitment of the Deep-Sea Benthos. Columbia University Press. pp. 179–194. ISBN 0231080042.
- Ruppert, Fox & Barnes 2004, pp. 887–888.
- Ruppert, Fox & Barnes 2004, p. 895.
- Ruppert, Fox & Barnes 2004, p. 888.
- Ruppert, Fox & Barnes 2004, p. 908.
- Ruppert, Fox & Barnes 2004, p. 916.
- Ruppert, Fox & Barnes 2004, p. 922.
- Yamaguchi, M.; J. S. Lucas (1984). "Natural parthenogenesis, larval and juvenile development, and geographical distribution of the coral reef asteroid Ophidiaster granifer". Marine Biology. 83 (1): 33–42. doi:10.1007/BF00393083. ISSN 0025-3162. S2CID 84475593.
- McGovern, Tamara M. (5 April 2002). "Patterns of sexual and asexual reproduction in the brittle star Ophiactis savignyi in the Florida Keys" (PDF). Marine Ecology Progress Series. 230: 119–126. Bibcode:2002MEPS..230..119M. doi:10.3354/meps230119.
- Monks, Sarah P. (1 April 1904). "Variability and Autotomy of Phataria". Proceedings of the Academy of Natural Sciences of Philadelphia. 56 (2): 596–600. ISSN 0097-3157. JSTOR 4063000.
- Kille, Frank R. (1942). "Regeneration of the Reproductive System Following Binary Fission in the Sea-Cucumber, Holothuria parvula (Selenka)" (PDF). Biological Bulletin. 83 (1): 55–66. doi:10.2307/1538013. ISSN 0006-3185. JSTOR 1538013. Retrieved 15 July 2011.
- Eaves, Alexandra A.; Palmer, A. Richard (11 September 2003). "Reproduction: Widespread cloning in echinoderm larvae". Nature. 425 (6954): 146. Bibcode:2003Natur.425..146E. doi:10.1038/425146a. hdl:10402/era.17195. ISSN 0028-0836. PMID 12968170. S2CID 4430104.
- Jaeckle, William B. (1 February 1994). "Multiple Modes of Asexual Reproduction by Tropical and Subtropical Sea Star Larvae: An Unusual Adaptation for Genet Dispersal and Survival" (PDF). Biological Bulletin. 186 (1): 62–71. doi:10.2307/1542036. ISSN 0006-3185. JSTOR 1542036. PMID 29283296. Retrieved 13 July 2011.
- Vaughn, Dawn (October 2009). "Predator-Induced Larval Cloning in the Sand Dollar Dendraster excentricus: Might Mothers Matter?". Biological Bulletin. 217 (2): 103–114. doi:10.1086/BBLv217n2p103. PMID 19875816. S2CID 26030855.
- McDonald, Kathryn A.; Dawn Vaughn (1 August 2010). "Abrupt Change in Food Environment Induces Cloning in Plutei of Dendraster excentricus". Biological Bulletin. 219 (1): 38–49. doi:10.1086/BBLv219n1p38. PMID 20813988. S2CID 24422314. Retrieved 16 July 2011.
- Vaughn, Dawn; Richard R. Strathmann (14 March 2008). "Predators induce cloning in echinoderm larvae". Science. 319 (5869): 1503. Bibcode:2008Sci...319.1503V. doi:10.1126/science.1151995. JSTOR 40284699. PMID 18339931. S2CID 206509992. Retrieved 16 July 2011.
- Vaughn, Dawn (March 2010). "Why run and hide when you can divide? Evidence for larval cloning and reduced larval size as an adaptive inducible defense". Marine Biology. 157 (6): 1301–1312. doi:10.1007/s00227-010-1410-z. ISSN 0025-3162. S2CID 84336247.
- Dorit, Walker & Barnes 1991, p. 778.
- van Egmond, Wim (1 July 2000). "Gallery of Echinoderm Larvae". Microscopy UK. Retrieved 2 February 2013.
- Xiaofeng, Wang; Hagdorn, Hans; Chuanshang, Wang (September 2006). "Pseudoplanktonic lifestyle of the Triassic crinoid Traumatocrinus from Southwest China". Lethaia. 39 (3): 187–193. doi:10.1080/00241160600715321.
- Pawson, David Leo. "Echinoderm: distribution and abundance". Encyclopædia Britannica. Retrieved 28 June 2013.
- Smith, J. E. (1937). "The structure and function of the tube feet in certain echinoderms" (PDF). Journal of the Marine Biological Association of the United Kingdom. 22 (1): 345–357. doi:10.1017/S0025315400012042. Archived from the original (PDF) on 15 November 2013.
- "Leather star - Dermasterias imbricata". Sea Stars of the Pacific Northwest. Archived from the original on 9 September 2012. Retrieved 27 September 2012.
- "Sand star - Luidia foliolata". Sea Stars of the Pacific Northwest. Archived from the original on 9 September 2012. Retrieved 26 September 2012.
- Ruppert, Fox & Barnes 2004, pp. 899–900.
- Ruppert, Fox & Barnes 2004, pp. 911–912.
- Messing, Charles. "The crinoid feeding mechanism". Charles Messing's Crinoid Pages. Retrieved 26 July 2012.
- Barnes, Robert D. (1982). Invertebrate Zoology. Holt-Saunders International. pp. 997–1007. ISBN 0-03-056747-5.
- Tom Carefoot. "Learn about feather stars: feeding & growth". A Snail's Odyssey. Archived from the original on 12 January 2013. Retrieved 23 February 2013.
- Ruppert, Fox & Barnes 2004, p. 893.
- Tom Carefoot. "Learn about sea urchins: feeding, nutrition & growth". A Snail's Odyssey. Archived from the original on 22 March 2013. Retrieved 23 February 2013.
- Tom Carefoot. "Learn about sand dollars: feeding & growth". A Snail's Odyssey. Archived from the original on 19 October 2012. Retrieved 23 February 2013.
- Ruppert, Fox & Barnes 2004, p. 914.
- Ruppert, Fox & Barnes 2004, pp. 884–885.
- Dorit, Walker & Barnes 1991, p. 779.
- Dorit, Walker & Barnes 1991, pp. 789–790.
- Mladenov, Philip V.; Igdoura, Suleiman; Asotra, Satish; Burke, Robert D. (1 April 1989). "Purification and partial characterization of an autotomy-promoting factor from the sea star Pycnopodia Helianthoides". The Biological Bulletin. 176 (2): 169–175. doi:10.2307/1541585. ISSN 0006-3185. JSTOR 1541585.
- Beamer, Victoria P. "Regeneration in Starfish (Asteroidea)". The Physiology of Arm Regeneration in Starfish (Asteroidea). Davidson College. Archived from the original on 9 April 2013. Retrieved 10 March 2013.
- Miller, John E. "Echinoderm: role in nature". Encyclopædia Britannica. Retrieved 28 June 2013.
- Herrera-Escalante, T; López-Pérez, R. A.; Leyte-Morales, G. E. (2005). "Bioerosion caused by the sea urchin Diadema mexicanum (Echinodermata: Echinoidea) at Bahías de Huatulco, Western Mexico". Revista de Biología Tropical. 53 (3): 263–273. PMID 17469255.
- Kaplan, M. (2010). "Sea stars suck up carbon". Nature. doi:10.1038/news.2009.1041.
- Osborne, Patrick L. (2000). Tropical Ecosystem and Ecological Concepts. Cambridge: Cambridge University Press. p. 464. ISBN 0-521-64523-9.
- Lawrence, J. M. (1975). "On the relationships between marine plants and sea urchins" (PDF). Oceanographic Marine Biological Annual Review. 13: 213–286. Archived from the original (PDF) on 5 February 2011.
- Birkeland, Charles; Lucas, John S. (1990). Acanthaster planci: Major Management Problem of Coral Reefs. Florida: CRC Press. ISBN 0849365996.
- Luciano, Brooke; Lyman, Ashleigh; McMillan, Selena; Nickels, Abby. "The symbiotic relationship between Sea cucumbers (Holothuriidae) and Pearlfish (Carapidae)". Retrieved 27 June 2013.
- Pangestuti, Ratih; Arifin, Zainal (2018). "Medicinal and health benefit effects of functional sea cucumbers". Journal of Traditional and Complementary Medicine. 8 (3): 341–351. doi:10.1016/j.jtcme.2017.06.007. ISSN 2225-4110. PMC 6035309. PMID 29992105.
- Sourced from the data reported in the FAO FishStat database
- "Sea Cucumbers Threatened by Asian Trade". The New York Times. Retrieved 1 April 2009.
- Wikisource:1911 Encyclopædia Britannica/Bêche-de-Mer
- Lawrence, John M. (2001). "The edible sea urchins". In John M. Lawrence (ed.). Edible Sea Urchins: Biology and Ecology. Developments in Aquaculture and Fisheries Science. 37. pp. 1–4. doi:10.1016/S0167-9309(01)80002-8. ISBN 9780444503909.
- Sartori D., Scuderi A.; Sansone G.; Gaion A. (February 2015). "Echinoculture: the rearing of Paracentrotus lividus in a recirculating aquaculture system—experiments of artificial diets for the maintenance of sexual maturation". Aquaculture International. 23: 111–125. doi:10.1007/s10499-014-9802-6. S2CID 18856183.
- "Insight from the Sea Urchin". Microscope Imaging Station. Exploratorium. Retrieved 4 February 2013.
- Sartori D., Gaion A. (2015). "Toxicity of polyunsaturated aldehydes of diatoms to Indo-Pacific bioindicator organism Echinometra mathaei". Drug and Chemical Toxicology. 39 (2): 124–8. doi:10.3109/01480545.2015.1041602. PMID 25945412. S2CID 207437380.
- Gaion A., Scuderi A.; Pellegrini D.; Sartori D. (2013). "Arsenic Exposure Affects Embryo Development of Sea Urchin, Paracentrotus lividus (Lamarck, 1816)". Bulletin of Environmental Contamination and Toxicology. 39 (2): 124–8. doi:10.3109/01480545.2015.1041602. PMID 25945412. S2CID 207437380.
- Longo, F. J.; Anderson, E. (June 1969). "Sperm differentiation in the sea urchins Arbacia punctulata and Strongylocentrotus purpuratus". Journal of Ultrastructure Research. 27 (5): 486–509. doi:10.1016/S0022-5320(69)80046-8. PMID 5816822.
- Barkhouse, C.; Niles, M.; Davidson, L. -A. (2007). "A literature review of sea star control methods for bottom and off bottom shellfish cultures" (PDF). Canadian Industry Report of Fisheries and Aquatic Sciences. 279 (7): 14–15. ISSN 1488-5409.
- "Sea stars". Wild Singapore. Retrieved 4 February 2013.
Cited texts
- Dorit, R. L.; Walker, W. F.; Barnes, R. D. (1991). Zoology, International edition. Saunders College Publishing. ISBN 978-0-03-030504-7.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. ISBN 81-315-0104-3.
Further reading
- Black, Rhona M. (1970). The Elements of Palaeontology. Cambridge University Press. ISBN 978-0-521-09615-7.
- Clark, A. M. (1968). Starfishes and their relations (2nd ed.). Trustees of the British Museum (Natural History).
- Clarkson, Euan Neilson Kerr (1993). Invertebrate palaeontology and evolution. ISBN 978-0-412-47990-8.
- Nichols, David (Ed.) (1969). Echinoderms. Ebury Press. ISBN 978-0-09-065994-4.CS1 maint: extra text: authors list (link)
- Paul, C. R. C.; Smith, A. B. (1984). "The early radiation and phylogeny of echinoderms". Biological Reviews. 59 (4): 443–481. doi:10.1111/j.1469-185X.1984.tb00411.x. S2CID 86572427.
- Shrock, R. R.; Twenhofel, W. H. (1953). Principles of Invertebrate Paleontology (2nd ed.). McGraw Hill. Chapter 14. LCC 52–5341.
- Smith, A. B. (2006). "The pre-radial history of echinoderms". Geological Journal. 40 (3): 255–280. doi:10.1002/gj.1018.
External links
| Wikispecies has information related to Echinodermata. |
| Wikimedia Commons has media related to Echinodermata. |
.jpg.webp)
