Hereditary multiple exostoses
Hereditary multiple osteochondromas (HMO), also known as hereditary multiple exostoses, is a disorder characterized by the development of multiple benign osteocartilaginous masses (exostoses) in relation to the ends of long bones of the lower limbs such as the femurs and tibias and of the upper limbs such as the humeri and forearm bones. They are also known as osteochondromas. Additional sites of occurrence include on flat bones such as the pelvic bone and scapula. The distribution and number of these exostoses show a wide diversity among affected individuals. Exostoses usually present during childhood. The vast majority of affected individuals become clinically manifest by the time they reach adolescence.[1][2] A small percentage of affected individuals are at risk for development of malignant transformation namely sarcomas. The incidence of hereditary multiple exostoses is around 1 in 50,000 individuals.[3] Hereditary multiple osteochondromas is the preferred term used by the World Health Organization.
| Hereditary multiple osteochondromas | |
|---|---|
| Other names | Hereditary multiple exostoses |
 | |
| Photograph of the legs of a 26-year-old male showing multiple lumps leading to deformity. | |
| Specialty | Medical genetics |
Presentation
A noticeable lump in relation to an extremity may be the first presenting symptom. Multiple deformities can arise, namely coronal plane deformities around the knees, ankles, shoulders, elbows, and wrists. For example, genu valgum (knock knees), ankle valgus, ulnar bowing and shortening, and radial head subluxation are encountered. The majority of affected individuals have clinically manifest osteochondromas around the knee. Forearm involvement in HMO is considerable.[1][4] Furthermore, short stature may occur and is generally disproportionate. Such manifestations usually result from disruption of physeal growth especially that osteochondromas typically arise at the metaphyseal ends of long bones in close proximity to the physis.[1][4] Intra-articular osteochondromas of the hip can induce limitation of range of motion, joint pain and acetabular dysplasia.[2] Likewise joint pain at other locations and neurovascular compression can occur. Furthermore, functional disability in regard to activities of daily living can be a presenting feature. Spinal deformity pain or neurological compromise should arouse suspicion of involvement of the vertebrae.[3]
Possible connection to autism
Some parents of children with HME have observed autism-like social problems in their children. To explore those observations more deeply, a 2012 study by the Sanford-Burnham Medical Research Institute used a mouse model of HME to observe cognitive function. The findings indicated that the mutant mice endorsed three autistic characteristics: social impairment, impairments in ultrasonic vocalization, and repetitive behavior.[5]
Genetics
HME is an autosomal dominant hereditary disorder. This means that a patient with HME has a 50% chance of transmitting this disorder to his or her children. Most individuals with HME have a parent who also has the condition, however, approximately 10% -20% of individuals with HME have the condition as a result of a spontaneous mutation and are thus the first person in their family to be affected.
HME has thus far been linked with mutations in three genes:
- EXT1 which maps to chromosome 8q24.1[6]
- EXT2 which maps to 11p13[7]
- EXT3 which maps to the short arm of Chromosome 19 (though its exact location has yet to be precisely determined)[8]
Mutations in these genes typically lead to the synthesis of a truncated EXT protein which does not function normally. It is known that EXT proteins are important enzymes in the synthesis of heparan sulfate; however the exact mechanism by which altered synthesis of heparan sulfate that could lead to the abnormal bone growth associated with HME is unclear. It is thought that normal chondrocyte proliferation and differentiation may be affected, leading to abnormal bone growth.[9][10] Since the HME genes are involved in the synthesis of a glycan (heparan sulfate), HME may be considered a congenital disorder of glycosylation according to the new CDG nomenclature suggested in 2009.[11]
For individuals with HME who are considering starting a family, preimplantation genetic testing and prenatal diagnosis are available to determine if their unborn child has inherited the disease. HME has a 96% penetrance, which means that if the affected gene is indeed transmitted to a child, the child will have a 96% of actually manifesting the disease, and 4% chance of having the disease but never manifesting it. The 96% penetrance figure comes from only one study.[12] Other studies have observed both incomplete and variable penetrance but without calculating the % penetrance, e.g.[13] In both the aforementioned studies the symptomless individuals carrying the faulty gene were predominantly female, leading to speculation that incomplete penetrance is more likely to be exhibited in females. Indeed, other work has shown that boys/men tend to have worse disease than females, as well as that the number of exostoses in affected members of the same family can vary greatly.[14] It is also possible for females to be severely affected. Severity of symptoms varies between individuals, even in the same family.
Symptoms are more likely to be severe if the mutation is on the ext1 gene rather than ext2 or ext3; ext1 is also the most commonly affected gene in patients of this disorder.[14]
Pathophysiology
It is characterized by the growth of cartilage-capped benign bone tumours around areas of active bone growth, particularly the metaphysis of the long bones. Typically five or six exostoses are found in upper and lower limbs. Most common locations are:[15]
HME can lead to the shortening and bowing of bones; affected individuals often have a short stature. Depending on their location the exostoses can cause the following problems: pain or numbness from nerve compression, vascular compromise, inequality of limb length, irritation of tendon and muscle, Madelung's deformity[16] as well as a limited range of motion at the joints upon which they encroach. A person with HME has an increased risk of developing a rare form of bone cancer called chondrosarcoma as an adult.[16] Problems may be had in later life and these could include weak bones and nerve damage.[17][18][19] The reported rate of transformation ranges from as low as 0.57%[12] to as high as 8.3% of people with HME.[20]
Diagnosis
The diagnosis of HMO is based upon establishing an accurate correlation between the above-mentioned clinical features and the characteristic radiographic features. Family history can provide an important clue to the diagnosis. This is supplemented by testing for the two genes in which pathogenic variants are known to cause HMO namely EXT1 and EXT2. A combination of sequence analysis and deletion analysis of the entire coding regions of both EXT1 and EXT2 detects pathogenic variants in 70–95% of affected individuals.[3][4] The hallmark of radiographic diagnosis is the presence of osteochondromas at the metaphyseal ends of long bones in which the cortex and medulla of the osteochondroma represent a continuous extension of the host bone. This is readily demonstrable in radiographs of the knees.[3][1]
Treatment
The indications for surgical intervention in individuals with HMO remain unclear and vary greatly across the medical literature. In general surgical treatment of HMO includes one or more of the following procedures: ostechondroma excision, gradual or acute bone lengthening such as the ulna lengthening, corrective osteotomies, temporary hemiepiphysiodesis to correct angular joint deformities such as distal radius hemiepiphysiodesis and medial distal tibial hemiepiphysiodesis.[1][3] Nevertheless, there is little evidence to support the ongoing pediatric orthopedic practice in hereditary multiple osteochondromas. Recent systematic reviews found insufficient evidence to prove that the ongoing surgical treatment of HMO improves function considerably or to prove that it impacts the quality of life of affected children.[1][2] To enhance the amount of evidence in the medical literature certain recommendations have been put forward. The construction of well-designed prospective studies that can provide a more clear relationship between surgical procedures, patient characteristics and outcomes is on high demand. Otherwise, following the current study designs will continue to raise more questions than answers.[1][2] Total hip arthroplasty has been used to remedy severe and painful HMO of the hip joint. Total hip arthroplasty in individuals with HMO is challenging because of distortion of anatomy and repeated surgeries performed to address complaints related to exostosis.[21]
Additional images
.jpg.webp) Multiple osteochondromas causing deformity of the forearm (shortening of the Radius with secondary bowing of the Ulna).
Multiple osteochondromas causing deformity of the forearm (shortening of the Radius with secondary bowing of the Ulna)..jpg.webp) multiple osteochondromas at the pelvis
multiple osteochondromas at the pelvis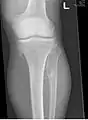 multiple osteochondromas around the knee
multiple osteochondromas around the knee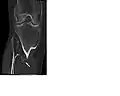 CT of osteochondroma in MO
CT of osteochondroma in MO
References
- EL-Sobky, TA; Samir, S; Atiyya, AN; Mahmoud, S; Aly, AS; Soliman, R (21 March 2018). "Current paediatric orthopaedic practice in hereditary multiple osteochondromas of the forearm: a systematic review". Sicot-J. 4: 10. doi:10.1051/sicotj/2018002. PMC 5863686. PMID 29565244.
- Makhdom, AM; Jiang, F; Hamdy, RC; Benaroch, TE; Lavigne, M; Saran, N (20 May 2014). "Hip joint osteochondroma: systematic review of the literature and report of three further cases". Advances in Orthopedics. 2014: 180254. doi:10.1155/2014/180254. PMC 4054980. PMID 24963411.
- Wuyts, W; Schmale, GA; Chansky, HA; et al. (21 November 2013). "Hereditary Multiple Osteochondromas". GeneReviews. University of Washington, Seattle. Retrieved 24 March 2018.
- Alvarez, CM; De Vera, MA; Heslip, TR; Casey, B (September 2007). "Evaluation of the anatomic burden of patients with hereditary multiple exostoses". Clin Orthop Relat Res. 462: 73–79. doi:10.1097/BLO.0b013e3181334b51. PMID 17589361. S2CID 39999620.
- Irie F, Badie-Mahdavi H, Yamaguchi Y (March 2012). "Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate". Proceedings of the National Academy of Sciences of the United States of America. 109 (13): 5052–6. Bibcode:2012PNAS..109.5052I. doi:10.1073/pnas.1117881109. PMC 3323986. PMID 22411800.
- Cook A, Raskind W, Blanton SH, Pauli RM, Gregg RG, Francomano CA, Puffenberger E, Conrad EU, Schmale G, Schellenberg G (1993). "Genetic heterogeneity in families with hereditary multiple exostoses". American Journal of Human Genetics. 53 (1): 71–9. PMC 1682231. PMID 8317501.
- Wu YQ, Heutink P, de Vries BB, Sandkuijl LA, van den Ouweland AM, Niermeijer MF, Galjaard H, Reyniers E, Willems PJ, Halley DJ (1994). "Assignment of a second locus for multiple exostoses to the pericentromeric region of chromosome 11". Human Molecular Genetics. 3 (1): 167–71. doi:10.1093/hmg/3.1.167. PMID 8162019.
- Le Merrer M, Legeai-Mallet L, Jeannin PM, Horsthemke B, Schinzel A, Plauchu H, Toutain A, Achard F, Munnich A, Maroteaux P (1994). "A gene for hereditary multiple exostoses maps to chromosome 19p". Human Molecular Genetics. 3 (5): 717–22. CiteSeerX 10.1.1.1028.5356. doi:10.1093/hmg/3.5.717. PMID 8081357.
- Zak BM, Crawford BE, Esko JD (2002). "Hereditary multiple exostoses and heparan sulfate polymerization". Biochimica et Biophysica Acta (BBA) - General Subjects. 1573 (3): 346–55. doi:10.1016/S0304-4165(02)00402-6. PMID 12417417.
- Stieber JR, Dormans JP (2005). "Manifestations of hereditary multiple exostoses". The Journal of the American Academy of Orthopaedic Surgeons. 13 (2): 110–20. doi:10.5435/00124635-200503000-00004. PMID 15850368. S2CID 29077708.
- Jaeken J, Hennet T, Matthijs G, Freeze HH (2009). "CDG nomenclature: time for a change!". Biochim. Biophys. Acta. 1792 (9): 825–6. doi:10.1016/j.bbadis.2009.08.005. PMC 3917312. PMID 19765534.
- Legeai-Mallet L, Munnich A, Maroteaux P, Le Merrer M (July 1997). "Incomplete penetrance and expressivity skewing in hereditary multiple exostoses". Clinical Genetics. 52 (1): 12–6. doi:10.1111/j.1399-0004.1997.tb02508.x. PMID 9272707.
- Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH, et al. (August 2004). "Novel mutations in the EXT1 gene in two consanguineous families affected with multiple hereditary exostoses (familial osteochondromatosis)". Clinical Genetics. 66 (2): 144–51. doi:10.1111/j.1399-0004.2004.00275.x. PMID 15253765.
- Porter DE, Lonie L, Fraser M, et al. (September 2004). "Severity of disease and risk of malignant change in hereditary multiple exostoses. A genotype-phenotype study". The Journal of Bone and Joint Surgery. British Volume. 86 (7): 1041–6. doi:10.1302/0301-620x.86b7.14815. hdl:20.500.11820/8754788e-ceea-4613-8e65-60d34fcf9edc. PMID 15446535.
- Turek's orthopaedics principles and their application (6th ed.). Philadelphia: Lippincott Williams & Wilkins. 2005. p. 263. ISBN 9780781742986.
- Davies, A. Mark; Pettersson, Holger (2002). Pettersson, Holger; Ostensen, Harald (eds.). Radiography of the Musculoskeletal System (PDF). Geneva: World Health Organization. pp. 177, 189. ISBN 978-92-4-154555-6.
- CANNON JF (1954). "Hereditary multiple exostoses". American Journal of Human Genetics. 6 (4): 419–25. PMC 1716573. PMID 14349947.
- McBride WZ (September 1988). "Hereditary multiple exostoses". American Family Physician. 38 (3): 191–2. PMID 3046271.
- Schmale GA, Conrad EU, Raskind WH (July 1994). "The natural history of hereditary multiple exostoses". The Journal of Bone and Joint Surgery. American Volume. 76 (7): 986–92. doi:10.2106/00004623-199407000-00005. PMID 8027127. Archived from the original on 2014-09-20.
- Kivioja A, Ervasti H, Kinnunen J, Kaitila I, Wolf M, Böhling T (March 2000). "Chondrosarcoma in a family with multiple hereditary exostoses". The Journal of Bone and Joint Surgery. British Volume. 82 (2): 261–6. doi:10.1302/0301-620X.82B2.0820261. PMID 10755438.
- Vaishya, R; Swami, S; Vijay, V; Vaish, A (5 Jan 2015). "Bilateral total hip arthroplasty in a young man with hereditary multiple exostoses". BMJ Case Rep. 2015: bcr2014207853. doi:10.1136/bcr-2014-207853. PMC 4289752. PMID 25564594.
External links
| Classification | |
|---|---|
| External resources |