Iodine pentoxide
Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid, and the only stable oxide of iodine. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:[1]
- 2HIO3 → I2O5 + H2O
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Iodine pentoxide | |
| Other names
Iodine(V) oxide Iodic anhydride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.031.569 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| I 2O 5 | |
| Molar mass | 333.81 g/mol |
| Appearance | white crystalline solid[1] hygroscopic |
| Density | 4.980 g/cm3[1] |
| Melting point | 300 °C (572 °F; 573 K)[2] (decomposes) |
| Solubility | soluble in water and nitric acid; insoluble in ethanol, ether and CS2 |
| −79.4·10−6 cm3/mol | |
| Hazards | |
| Main hazards | oxidizer |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
iodine pentafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
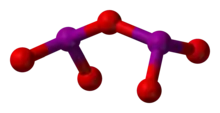
Structure
I2O5 is bent with an I–O–I angle of 139.2°, but the molecule has no mirror plane so its symmetry is C2 rather than C2v. The terminal I–O distances are around 1.80 Å and the bridging I–O distances are around 1.95 Å.[3]
Reactions
Iodine pentoxide easily oxidises carbon monoxide to carbon dioxide at room temperature:
- 5 CO + I2O5 → I2 + 5 CO2
This reaction can be used to analyze the concentration of CO in a gaseous sample.
I2O5 forms iodyl salts, [IO2+], with SO3 and S2O6F2, but iodosyl salts, [IO+], with concentrated sulfuric acid.
Iodine pentoxide decomposes to iodine (vapor) and oxygen when heated to about 350 °C.[4]
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 851–852. ISBN 978-0-08-037941-8.
- Patnaik, P. (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- Selte, K.; Kjekshus, A. (1970). "Iodine Oxides: Part III. The Crystal Structure of I2O5" (pdf). Acta Chemica Scandinavica. 24 (6): 1912–1924. doi:10.3891/acta.chem.scand.24-1912.
- G. Baxter and G. Tilley, "A Revision of the Atomic Weights of Iodine and Silver," The Chemical News and Journal of Industrial Science; Volumes 99-100, Royal Society Anniversary Meeting, December 3, 1909, p. 276. (Google Books)
