Metal nitrosyl complex
Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal.[1] Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
Bonding and structure

Most complexes containing the NO ligand can be viewed as derivatives of the nitrosyl cation, NO+. The nitrosyl cation is isoelectronic with carbon monoxide, thus the bonding between a nitrosyl ligand and a metal follows the same principles as the bonding in carbonyl complexes. The nitrosyl cation serves as a two-electron donor to the metal and accepts electrons from the metal via back-bonding. The compounds Co(NO)(CO)3 and Ni(CO)4 illustrate the analogy between NO+ and CO. In an electron-counting sense, two linear NO ligands are equivalent to three CO groups. This trend is illustrated by the isoelectronic pair Fe(CO)2(NO)2 and [Ni(CO)4].[2] These complexes are isoelectronic and, incidentally, both obey the 18-electron rule. The formal description of nitric oxide as NO+ does not match certain measureable and calculated properties. In an alternative description, nitric oxide serves as a 3-electron donor, and the metal-nitrogen interaction is a triple bond.
Linear vs bent nitrosyl ligands
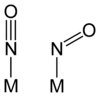
The M-N-O unit in nitrosyl complexes is usually linear, or no more than 15° from linear. In some complexes, however, especially when back-bonding is less important, the M-N-O angle can strongly deviate from 180°. Linear and bent NO ligands can be distinguished using infrared spectroscopy. Linear M-N-O groups absorb in the range 1650–1900 cm−1, whereas bent nitrosyls absorb in the range 1525–1690 cm−1. The differing vibrational frequencies reflect the differing N-O bond orders for linear (triple bond) and bent NO (double bond).
The bent NO ligand is sometimes described as the anion, NO−. Prototypes for such compounds are the organic nitroso compounds, such as nitrosobenzene. A complex with a bent NO ligand is trans-[Co(en)2(NO)Cl]+.
The adoption of linear vs bent bonding can be analyzed with the Enemark-Feltham notation.[3] In their framework, the factor that determines the bent vs linear NO ligands is the sum of electrons of pi-symmetry. Complexes with "pi-electrons" in excess of 6 tend to have bent NO ligands. Thus, [Co(en)2(NO)Cl]+, with seven electrons of pi-symmetry (six in t2g orbitals and one on NO), adopts a bent NO ligand, whereas [Fe(CN)5(NO)]3−, with six electrons of pi-symmetry, adopts a linear nitrosyl. In a further illustration, the {MNO} d-electron count of the [Cr(CN)5NO]3− anion is shown. In this example, the cyanide ligands are "innocent", i.e., they have a charge of −1 each, −5 total. To balance the fragment's overall charge, the charge on {CrNO} is thus +2 (−3 = −5 + 2). Using the neutral electron counting scheme, Cr has 6 d electrons and NO· has one electron for a total of 7. Two electrons are subtracted to take into account that fragment's overall charge of +2, to give 5. Written in the Enemark-Feltham notation, the d electron count is {CrNO}5. The results are the same if the nitrosyl ligand were considered NO+ or NO−.[3]
Bridging nitrosyl ligands
Nitric oxide can also serve as a bridging ligand. In the compound [Mn3(η5C5H5)3 (μ2-NO)3 (μ3-NO)], three NO groups bridge two metal centres and one NO group bridge to all three.[2]
Representative classes of compounds
Homoleptic nitrosyl complexes
Metal complexes containing only nitrosyl ligands are called isoleptic nitrosyls. They are rare, the premier member being Cr(NO)4.[4] Even trinitrosyl complexes are uncommon, whereas polycarbonyl complexes are routine.
Roussin red and black salts
One of the earliest examples of a nitrosyl complex to be synthesized is Roussin's red salt, which is a sodium salt of the anion [Fe2(NO)4S2]2−. The structure of the anion can be viewed as consisting of two tetrahedra sharing an edge. Each iron atom is bonded linearly to two NO+ ligands and shares two bridging sulfido ligands with the other iron atom. Roussin's black salt has a more complex cluster structure. The anion in this species has the formula [Fe4(NO)7S3]−. It has C3v symmetry. It consists of a tetrahedron of iron atoms with sulfide ions on three faces of the tetrahedron. Three iron atoms are bonded to two nitrosyl groups. The iron atom on the threefold symmetry axis has a single nitrosyl group which also lies on that axis.
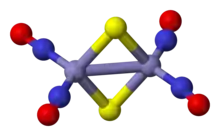 The anion in Roussin's Red Salt, [Fe2S2(NO)4]2−.
The anion in Roussin's Red Salt, [Fe2S2(NO)4]2−.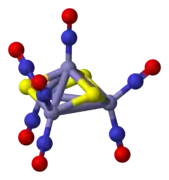 The anion in Roussin's black salt, [Fe4S3(NO)7]−.
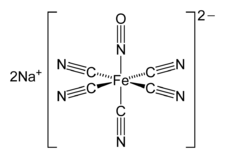
The anion in Roussin's black salt, [Fe4S3(NO)7]−.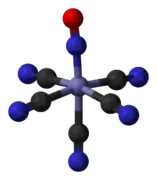 The nitroprusside anion, [Fe(CN)5NO]2−, an octahedral complex containing a "linear NO" ligand.
The nitroprusside anion, [Fe(CN)5NO]2−, an octahedral complex containing a "linear NO" ligand.2NO-3D-balls.png.webp) trans-[Co(en)2(NO)Cl]+, an octahedral complex containing a "bent NO" ligand.
trans-[Co(en)2(NO)Cl]+, an octahedral complex containing a "bent NO" ligand.
Preparation
Nitrosyl complexes are typically prepared by treating reduced metal complexes with nitric oxide. The nitrosylation of cobalt carbonyl is illustrative:[5]
- Co2(CO)8 + 2 NO → 2 CoNO(CO)3 + 2 CO
From nitrosonium sources
Replacement of ligands by the nitrosyl cation may be accomplished using nitrosyl tetrafluoroborate, [NO]BF4. When applied to the hexacarbonyls of molybdenum and tungsten, the NO binds to the metal:[6][7]
- M(CO)6 + 4 MeCN + 2 NOBF4 → [M(NO)2(MeCN)4](BF4)2
Nitrosyl chloride and molybdenum hexacarbonyl react to give [Mo(NO)2Cl2]n.[8] Diazald is also used as an NO source.[9]
Other methods
Other indirect methods are indirect with the NO group deriving from some other species, often accompanied by oxidation and reduction reactions. A classic example is provided by the brown ring test in which the nitrate ion is the source of a nitric oxide ligand.
Reactions
An important reaction is the acid/base equilibrium:
- [LnMNO]2+ + 2OH− ⇌ LnMNO2 + H2O
This equilibrium serves to confirm that the linear nitrosyl ligand is, formally, NO+, with nitrogen in the oxidation state +3
- NO+ + 2 OH− ⇌ NO2− + H2O
Since nitrogen is more electronegative than carbon, metal-nitrosyl complexes tend to be more electrophilic than related metal carbonyl complexes. Nucleophiles often add to the nitrogen.[1] The nitrogen atom in bent metal nitrosyls is basic, thus can be oxidized, alkylated, and protonated, e.g.:
- (Ph3P)2(CO)ClOsNO + HCl → (Ph3P)2(CO)ClOsN(H)O
In rare cases, NO is cleaved by metal centers:
- Cp2NbMe2 + NO → Cp2(Me)Nb(O)NMe
- 2 Cp2(Me)Nb(O)NMe → 2 Cp2Nb(O)Me + ½MeN=NMe
Applications
Metal-nitrosyls are assumed to be intermediates in catalytic converters, which reduce the emission of NOx from internal combustion engines. This application has been described as “one of the most successful stories in the development of catalysts.”[11]
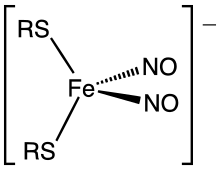
Metal-catalyzed reactions of NO are not often useful in organic chemistry. In biology and medicine, nitric oxide is however an important signalling molecule in nature and this fact is the basis of the most important applications of metal nitrosyls. The nitroprusside anion, [Fe(CN)5NO]2−, a mixed nitrosyl cyano complex, has pharmaceutical applications as a slow release agent for NO. The signalling function of NO is effected via its complexation to haeme proteins, where it binds in the bent geometry. Nitric oxide also attacks iron-sulfur proteins giving dinitrosyl iron complexes.
References
- Hayton, T. W.; Legzdins, P.; Sharp, W. B. (2002). "Coordination and Organometallic Chemistry of Metal-NO Complexes". Chem. Rev. 102 (1): 935–991. doi:10.1021/cr000074t. PMID 11942784.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 447–453. ISBN 978-0-08-037941-8.
- Enemark, J. H.; Feltham, R. D. (1974). "Principles of structure, bonding, and reactivity for metal nitrosyl complexes". Coord. Chem. Rev. 1974 (13): 339–406. doi:10.1016/S0010-8545(00)80259-3.
- Herberhold Max (1972). "Tetranitrosylchromium [Cr(NO)4]". Angewandte Chemie International Edition in English. 11: 1092–1094. doi:10.1002/anie.197210921.
- Paul Gilmont Arthur A. Blanchard (1946). "Dicobalt Octacarbonyl, Cobalt Nitrosyl Tricarbonyl, and Cobalt Tetracarbomyl Hydride". Inorg. Synth. 2: 238. doi:10.1002/9780470132333.ch76.CS1 maint: uses authors parameter (link)
- Richard R. Thomas, Ayusman Sen (1990). "Acetonitrile Complexes of Selected Transition Metal Cations". Inorg. Synth. 28: 63–67. doi:10.1002/9780470132593.ch14.CS1 maint: uses authors parameter (link)
- Francine Agbossou Edward J. O'Connor Charles M. Garner N. Quirós Méndez Jesús M. Fernández Alan T. Patton James A. Ramsden J. A. Gladysz (1992). "Cyclopentadienyl Rhenium Complexes". Inorg. Synth. 29: 211. doi:10.1002/9780470132609.ch51.CS1 maint: uses authors parameter (link)
- B. F. G. Johnson K. H. Al‐Obadi (1970). "Dihalogenodinitrosylmolybdenum and Dihalogenodinitrosyltungsten". Inorg. Synth. 12: 264. doi:10.1002/9780470132432.ch47.CS1 maint: uses authors parameter (link)
- James K. Hoyano, Peter Legzdins, John T. Malito (1978). "(η5‐Cyclopentadienydnitrosyl Complexes of Chromium, Molybdenum, and Tungsten". Inorg. Synth. 13: 126. doi:10.1002/9780470132494.ch21.CS1 maint: uses authors parameter (link)
- Walker, F. A. (2005). "Nitric Oxide Interaction with Insect Nitrophorins and Thoughts on the Electron Configuration of the FeNO6 Complex". J. Inorg. Biochem. 99: 216–236. doi:10.1016/j.jinorgbio.2004.10.009. PMID 15598503.
- Kaspar, Jan; Fornasiero, Paolo; Hickey, Neal (2003). "Automotive Catalytic Converters: Current Status and Some Perspectives". Catalysis Today. 77: 419–449. doi:10.1016/S0920-5861(02)00384-X.CS1 maint: uses authors parameter (link)
- Jessica Fitzpatrick, Eunsuk Kim (2015). "Synthetic Modeling Chemistry of Iron–Sulfur Clusters in Nitric Oxide Signaling". Acc. Chem. Res. 48: 2453–2461. doi:10.1021/acs.accounts.5b00246. PMID 26197209.CS1 maint: uses authors parameter (link)