Pancreatic lipase family
Triglyceride lipases (EC 3.1.1.3) are a family of lipolytic enzymes that hydrolyse ester linkages of triglycerides.[1] Lipases are widely distributed in animals, plants and prokaryotes.
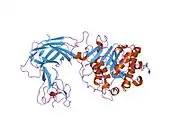
 Complex of human pancreatic lipase with colipase | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Lipase | ||||||||
| Pfam | PF00151 | ||||||||
| InterPro | IPR013818 | ||||||||
| PROSITE | PDOC00110 | ||||||||
| SCOP2 | 1lpa / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 127 | ||||||||
| OPM protein | 1lpa | ||||||||
| |||||||||
At least three tissue-specific isozymes exist in higher vertebrates, pancreatic, hepatic and gastric/lingual. These lipases are closely related to each other and to lipoprotein lipase (EC 3.1.1.34), which hydrolyses triglycerides of chylomicrons and very low density lipoproteins (VLDL).[2]
The most conserved region in all these proteins is centred on a serine residue which has been shown[3] to participate, with a histidine and an aspartic acid residue, in a charge relay system. Such a region is also present in lipases of prokaryotic origin and in lecithin-cholesterol acyltransferase (EC 2.3.1.43) (LCAT),[4] which catalyzes fatty acid transfer between phosphatidylcholine and cholesterol.
Human pancreatic lipase
Pancreatic lipase, also known as pancreatic triacylglycerol lipase or steapsin, is an enzyme secreted from the pancreas. As the primary lipase enzyme that hydrolyzes (breaks down) dietary fat molecules in the human digestive system, it is one of the main digestive enzymes, converting triglyceride substrates like 1 found in ingested oils to monoglycerides 3 and free fatty acids 2a and 2b.[5]
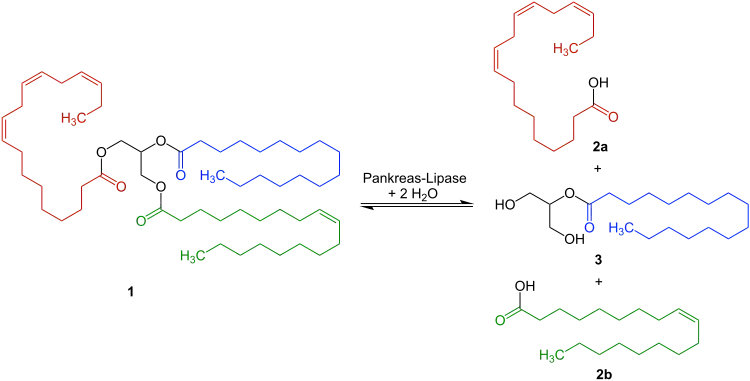
Bile salts secreted from the liver and stored in gallbladder are released into the duodenum, where they coat and emulsify large fat droplets into smaller droplets, thus increasing the overall surface area of the fat, which allows the lipase to break apart the fat more effectively. The resulting monomers (2 free fatty acids and one 2-monoacylglycerol) are then moved by way of peristalsis along the small intestine to be absorbed into the lymphatic system by a specialized vessel called a lacteal.
Unlike some pancreatic enzymes that are activated by proteolytic cleavage (e.g., trypsinogen), pancreatic lipase is secreted in its final form. However, it becomes efficient only in the presence of colipase in the duodenum.
In humans, pancreatic lipase is encoded by the PNLIP gene.[6][7]
Human proteins containing this domain
Diagnostic importance
Pancreatic lipase is secreted into the duodenum through the duct system of the pancreas. Its concentration in serum is normally very low. Under extreme disruption of pancreatic function, such as pancreatitis or pancreatic adenocarcinoma, the pancreas may begin to autolyse and release pancreatic enzymes including pancreatic lipase into serum. Thus, through measurement of serum concentration of pancreatic lipase, acute pancreatitis can be diagnosed.[8]
References
- Chapus C, Rovery M, Sarda L, Verger R (1988). "Minireview on pancreatic lipase and colipase". Biochimie. 70 (9): 1223–1234. doi:10.1016/0300-9084(88)90188-5. PMID 3147715.
- Persson B, Bengtsson-Olivecrona G, Enerback S, Olivecrona T, Jornvall H (1989). "Structural features of lipoprotein lipase. Lipase family relationships, binding interactions, non-equivalence of lipase cofactors, vitellogenin similarities and functional subdivision of lipoprotein lipase". Eur. J. Biochem. 179 (1): 39–45. doi:10.1111/j.1432-1033.1989.tb14518.x. PMID 2917565.
- Blow D (1990). "Enzymology. More of the catalytic triad". Nature. 343 (6260): 694–695. Bibcode:1990Natur.343..694B. doi:10.1038/343694a0. PMID 2304545. S2CID 4281247.
- McLean J, Fielding C, Drayna D, Dieplinger H, Baer B, Kohr W, Henzel W, Lawn R (1986). "Cloning and expression of human lecithin-cholesterol acyltransferase cDNA". Proc. Natl. Acad. Sci. U.S.A. 83 (8): 2335–2339. Bibcode:1986PNAS...83.2335M. doi:10.1073/pnas.83.8.2335. PMC 323291. PMID 3458198.
- Peter Nuhn: Naturstoffchemie, S. Hirzel Wissenschaftliche Verlagsgesellschaft, Stuttgart, 2. Auflage, 1990, S. 308−309, ISBN 3-7776-0473-9.
- Davis RC, Diep A, Hunziker W, Klisak I, Mohandas T, Schotz MC, Sparkes RS, Lusis AJ (December 1991). "Assignment of human pancreatic lipase gene (PNLIP) to chromosome 10q24-q26". Genomics. 11 (4): 1164–6. doi:10.1016/0888-7543(91)90048-J. PMID 1783385.
- "Entrez Gene: pancreatic lipase".
- Koop H (September 1984). "Serum levels of pancreatic enzymes and their clinical significance". Clin Gastroenterol. 13 (3): 739–61. PMID 6207965.
- "US orlistat label" (PDF). FDA. August 2015. Retrieved 18 April 2018. For label updates see FDA index page for NDA 020766
- Lunder, M.; Bratkovič, T.; Kreft, S.; Štrukelj, B. (2005). "Peptide inhibitor of pancreatic lipase selected by phage display using different elution strategies". Journal of Lipid Research. 46 (7): 1512–1516. doi:10.1194/jlr.M500048-JLR200. PMID 15863836.
Further reading
- Roussel A, Yang Y, Ferrato F, Verger R, Cambillau C, Lowe M (November 1998). "Structure and activity of rat pancreatic lipase-related protein 2". J. Biol. Chem. 273 (48): 32121–8. doi:10.1074/jbc.273.48.32121. PMID 9822688.
- Crandall WV, Lowe ME (2001). "Colipase residues Glu64 and Arg65 are essential for normal lipase-mediated fat digestion in the presence of bile salt micelles". J. Biol. Chem. 276 (16): 12505–12. doi:10.1074/jbc.M009986200. PMID 11278590.
- Freie AB, Ferrato F, Carrière F, Lowe ME (2006). "Val-407 and Ile-408 in the beta5'-loop of pancreatic lipase mediate lipase-colipase interactions in the presence of bile salt micelles". J. Biol. Chem. 281 (12): 7793–800. doi:10.1074/jbc.M512984200. PMC 3695395. PMID 16431912.
- Hegele RA, Ramdath DD, Ban MR, Carruthers MN, Carrington CV, Cao H (2001). "Polymorphisms in PNLIP, encoding pancreatic lipase, and associations with metabolic traits". J. Hum. Genet. 46 (6): 320–4. doi:10.1007/s100380170066. PMID 11393534.
- Chahinian H, Sias B, Carrière F (2000). "The C-terminal domain of pancreatic lipase: functional and structural analogies with c2 domains". Curr. Protein Pept. Sci. 1 (1): 91–103. doi:10.2174/1389203003381487. PMID 12369922.
- Ranaldi S, Belle V, Woudstra M, Rodriguez J, Guigliarelli B, Sturgis J, Carriere F, Fournel A (2009). "Lid opening and unfolding in human pancreatic lipase at low pH revealed by site-directed spin labeling EPR and FTIR spectroscopy". Biochemistry. 48 (3): 630–8. doi:10.1021/bi801250s. PMID 19113953.
- Grupe A, Li Y, Rowland C, Nowotny P, Hinrichs AL, Smemo S, Kauwe JS, Maxwell TJ, Cherny S, Doil L, Tacey K, van Luchene R, Myers A, Wavrant-De Vrièze F, Kaleem M, Hollingworth P, Jehu L, Foy C, Archer N, Hamilton G, Holmans P, Morris CM, Catanese J, Sninsky J, White TJ, Powell J, Hardy J, O'Donovan M, Lovestone S, Jones L, Morris JC, Thal L, Owen M, Williams J, Goate A (2006). "A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease". Am. J. Hum. Genet. 78 (1): 78–88. doi:10.1086/498851. PMC 1380225. PMID 16385451.
- Thomas A, Allouche M, Basyn F, Brasseur R, Kerfelec B (2005). "Role of the lid hydrophobicity pattern in pancreatic lipase activity". J. Biol. Chem. 280 (48): 40074–83. doi:10.1074/jbc.M502123200. PMID 16179352.
- van Tilbeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C (1993). "Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography". Nature. 362 (6423): 814–20. Bibcode:1993Natur.362..814V. doi:10.1038/362814a0. PMID 8479519. S2CID 4305832.
- Lessinger JM, Arzoglou P, Ramos P, Visvikis A, Parashou S, Calam D, Profilis C, Férard G (2003). "Preparation and characterization of reference materials for human pancreatic lipase: BCR 693 (from human pancreatic juice) and BCR 694 (recombinant)". Clin. Chem. Lab. Med. 41 (2): 169–76. doi:10.1515/CCLM.2003.028. PMID 12667003. S2CID 28593258.
- Colin DY, Deprez-Beauclair P, Allouche M, Brasseur R, Kerfelec B (2008). "Exploring the active site cavity of human pancreatic lipase". Biochem. Biophys. Res. Commun. 370 (3): 394–8. doi:10.1016/j.bbrc.2008.03.043. PMID 18353248.
- Ramos P, Coste T, Piémont E, Lessinger JM, Bousquet JA, Chapus C, Kerfelec B, Férard G, Mély Y (2003). "Time-resolved fluorescence allows selective monitoring of Trp30 environmental changes in the seven-Trp-containing human pancreatic lipase". Biochemistry. 42 (43): 12488–96. doi:10.1021/bi034900e. PMID 14580194.
- Yang Y, Lowe ME (1998). "Human pancreatic triglyceride lipase expressed in yeast cells: purification and characterization". Protein Expr. Purif. 13 (1): 36–40. doi:10.1006/prep.1998.0874. PMID 9631512.
- Sims HF, Jennens ML, Lowe ME (1993). "The human pancreatic lipase-encoding gene: structure and conservation of an Alu sequence in the lipase gene family". Gene. 131 (2): 281–5. doi:10.1016/0378-1119(93)90307-O. PMID 8406023.
- Grandval P, De Caro A, De Caro J, Sias B, Carrière F, Verger R, Laugier R (2004). "Critical evaluation of a specific ELISA and two enzymatic assays of pancreatic lipases in human sera". Pancreatology. 4 (6): 495–503, discussion 503–4. doi:10.1159/000080246. PMID 15316225. S2CID 39583651.
- Belle V, Fournel A, Woudstra M, Ranaldi S, Prieri F, Thomé V, Currault J, Verger R, Guigliarelli B, Carrière F (2007). "Probing the opening of the pancreatic lipase lid using site-directed spin labeling and EPR spectroscopy". Biochemistry. 46 (8): 2205–14. doi:10.1021/bi0616089. PMID 17269661.
- Lowe ME (1997). "Structure and function of pancreatic lipase and colipase". Annu. Rev. Nutr. 17: 141–58. doi:10.1146/annurev.nutr.17.1.141. PMID 9240923.
- Bourbon-Freie A, Dub RE, Xiao X, Lowe ME (2009). "Trp-107 and trp-253 account for the increased steady state fluorescence that accompanies the conformational change in human pancreatic triglyceride lipase induced by tetrahydrolipstatin and bile salt". J. Biol. Chem. 284 (21): 14157–64. doi:10.1074/jbc.M901154200. PMC 2682864. PMID 19346257.
- Ranaldi S, Belle V, Woudstra M, Bourgeas R, Guigliarelli B, Roche P, Vezin H, Carrière F, Fournel A (2010). "Amplitude of pancreatic lipase lid opening in solution and identification of spin label conformational subensembles by combining continuous wave and pulsed EPR spectroscopy and molecular dynamics". Biochemistry. 49 (10): 2140–9. doi:10.1021/bi901918f. PMID 20136147.