Pregnenolone sulfate
Pregnenolone sulfate (PS, PREGS) is an endogenous excitatory neurosteroid that is synthesized from pregnenolone.[1][2] It is known to have cognitive and memory-enhancing, antidepressant, anxiogenic, and proconvulsant effects.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(3S,8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate | |
| Other names
Pregn-sulf; Pregnenolone monosulfate; Pregnenolone hydrogen sulfate; Pregnenolone 3β-sulfate; 5-Pregnen-3β-ol-20-one sulfate; (3β)-3-(Sulfooxy)pregn-5-en-20-one; 5-Pregnen-3β-sulfate-20-one; 20-Oxo-5-pregnen-3β-yl sulfate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H32O5S | |
| Molar mass | 396.54 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biological activity
Pregnenolone sulfate is a neurosteroid with excitatory effects in the brain, acting as a potent negative allosteric modulator of the GABAA receptor and a weak positive allosteric modulator of the NMDA receptor.[1][2] To a lesser extent, it also acts as a negative allosteric modulator of the AMPA, kainate, and glycine receptors,[3][4] and may interact with the nACh receptors as well.[1] In addition to its effects on ligand-gated ion channels, pregnenolone sulfate is an agonist of the sigma receptor,[2] as well as an activator of the TRPM1 and TRPM3 channels.[1] It may also interact with potassium channels and voltage-gated sodium channels[1] and has been found to inhibit voltage-gated calcium channels.[5]
Biochemistry
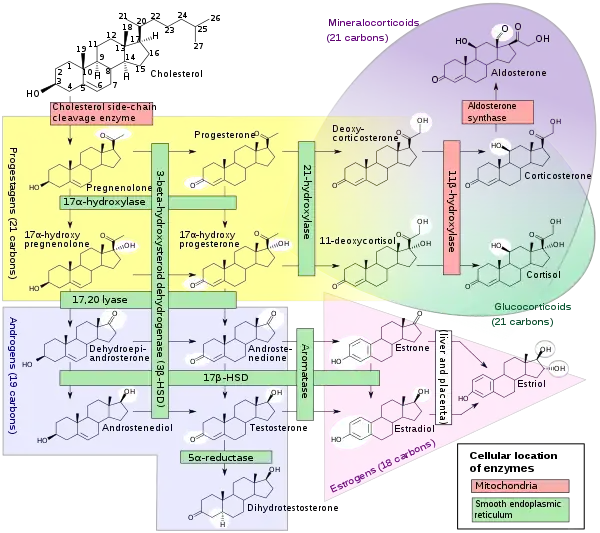
Biosynthesis
Pregnenolone sulfate is synthesized from pregnenolone via sulfation. Pregnenolone itself is produced from cholesterol via cholesterol side-chain cleavage enzyme.
Distribution
Pregnenolone sulfate does not cross the blood–brain barrier.[6][7]
Chemistry
Pregnenolone sulfate, also known as pregn-5-en-3β-ol-20-one 3β-sulfate, is a naturally occurring pregnane steroid and a derivative of cholesterol. It is the C3β sulfate ester of pregnenolone. A closely related steroid is dehydroepiandrosterone sulfate (DHEA-S), which is the C3β sulfate ester of dehydroepiandrosterone (DHEA).
References
- Harteneck C (2013). "Pregnenolone sulfate: from steroid metabolite to TRP channel ligand". Molecules. 18 (10): 12012–28. doi:10.3390/molecules181012012. PMID 24084011.
- Reddy DS (2010). "Neurosteroids: endogenous role in the human brain and therapeutic potentials". Prog. Brain Res. 186: 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. PMC 3139029. PMID 21094889.
- Park-Chung M, Wu FS, Farb DH (July 1994). "3 alpha-Hydroxy-5 beta-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons". Mol. Pharmacol. 46 (1): 146–50. PMID 7520124.
- Yaghoubi N, Malayev A, Russek SJ, Gibbs TT, Farb DH (August 1998). "Neurosteroid modulation of recombinant ionotropic glutamate receptors". Brain Res. 803 (1–2): 153–60. doi:10.1016/s0006-8993(98)00644-1. PMID 9729352.
- Mellon SH (2007). "Neurosteroid regulation of central nervous system development". Pharmacol. Ther. 116 (1): 107–24. doi:10.1016/j.pharmthera.2007.04.011. PMC 2386997. PMID 17651807.
- Jong Rho; Raman Sankar; Carl E. Stafstrom (18 June 2010). Epilepsy: Mechanisms, Models, and Translational Perspectives. CRC Press. pp. 479–. ISBN 978-1-4200-8560-0.
- CIBA Foundation Symposium (30 April 2008). Steroids and Neuronal Activity. John Wiley & Sons. pp. 101–. ISBN 978-0-470-51399-6.