Rhenium disulfide
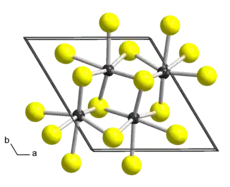
Rhenium disulfide is an inorganic compound of rhenium and sulfur with the formula ReS2. It has a layered structure where atoms are strongly bonded within each layer. The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
 | |
| Names | |
|---|---|
| IUPAC name
Bis(sulfanylidene)rhenium | |
| Other names
Rhenium(IV) sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.695 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ReS2 | |
| Molar mass | 250.337 g/mol[1] |
| Odor | odorless |
| Density | 7.6 g/cm3[1] |
| insoluble | |
| Structure | |
| Triclinic, aP12, space group P1, No 2[2] | |
a = 0.6455 nm, b = 0.6362 nm, c = 0.6401 nm α = 91.60°, β = 105.04°, γ = 118.97° | |
Formula units (Z) |
4 |
| Related compounds | |
Other anions |
Rhenium(IV) oxide Rhenium diselenide Rhenium ditelluride |
Other cations |
Manganese diselenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
ReS2 is found in nature as the minearl rheniite.[3] It can be synthesized from the reaction between rhenium and sulfur at 1000 °C, or the decomposition of rhenium(VII) sulfide at 1100 °C:[4]
- Re + 2 S → ReS2
- Re2S7 → 2 ReS2 + 3 S
Nanostructured ReS2 can usually be achieved through mechanical exfoliation, chemical vapor deposition (CVD), and chemical and liquid exfoliations. Larger crystals can be grown with the assistance of liquid carbonate flux at high pressure. It is widely used in electronic and optoelectronic device, energy storage, photocatalytic and electrocatalytic reactions.[5]
Properties
It is a two-dimensional (2D) group VII transition metal dichalcogenide (TMD). ReS2 was isolated down to monolayers which is only one unit cell in thickness for the first time in 2014.[6] These monolayers have shown layer-independent electrical, optical, and vibrational properties much different from other TMDs.
References
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.84. ISBN 1439855110.
- Wildervanck, J.C; Jellinek, F (1971). "The dichalcogenides of technetium and rhenium". Journal of the Less Common Metals. 24: 73–81. doi:10.1016/0022-5088(71)90168-8.
- Rheniite, MinDat.org, retrieved 2020-07-17
- Brauer, Georg (1981). Handbuch der Präparativen Anorganischen Chemie. Band III (in German) (3rd ed.). Stuttgart: Ferdinand Enke. p. 1619. ISBN 3-432-87823-0.
- Rahman, Mohammad; Davey, Kenneth; Qiao, Shi-Zhang (2017). "Advent of 2D Rhenium Disulfide (ReS2): Fundamentals to Applications" (PDF). Advanced Functional Materials. 27 (10): 1606129. doi:10.1002/adfm.201606129. hdl:2440/103880.
- Tongay, Sefaattin; Sahin, Hasan; Ko, Changhyun; Luce, Alex; Fan, Wen; Liu, Kai; Zhou, Jian; Huang, Ying-Sheng; Ho, Ching-Hwa; Yan, Jinyuan; Ogletree, D. Frank; Aloni, Shaul; Ji, Jie; Li, Shushen; Li, Jingbo; Peeters, F. M.; Wu, Junqiao (2014). "Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling". Nature Communications. 5. doi:10.1038/ncomms4252. PMID 24500082.