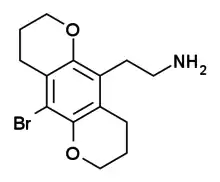
2C-B-BUTTERFLY
2C-B-BUTTERFLY is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, which was discovered in 1999 by Michael S. Whiteside and Aaron Monte.[1] It is a ring-expanded homologue of the better known compound 2C-B-FLY, and has similar properties as an agonist for serotonin receptors, but with more selectivity for 5-HT2C over 5-HT2A.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| Chemical and physical data | |
| Formula | C14H18BrNO2 |
| Molar mass | 312.207 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Analogues and derivatives
Analogues and derivatives of 2C-B:
25-N:
- 25B-N1POMe
- 25B-NAcPip
25-NM:
- 25B-NMe7BF
- 25B-NMe7BT
- 25B-NMe7Box
- 25B-NMe7DHBF
- 25B-NMe7Ind
- 25B-NMe7Indz
- 25B-NMePyr
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
Other:
- 2C-B-AN
- 2C-B-BUTTERFLY
- 2C-B-DRAGONFLY-NBOH
- 2C-B-DragonFLY
- 2C-B-FLY-NB2EtO5Cl
- 2CB-5-hemifly
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- TCB-2 (2C-BCB)
Legal Status
2C-B-BUTTERFLY is illegal in Latvia.[4]
See also
References
- Whiteside MS (1999). "Synthesis of hexahydrobenzodipyrans as ring-expanded analogues of potent serotonin 5-HT2A/2C receptor probes". UW-LaCrosseJUR. 2: 61–68. CiteSeerX 10.1.1.688.4722.
- Whiteside MS, Kurrasch-Orbaugh D, Marona-Lewicka D, Nichols DE, Monte A (October 2002). "Substituted hexahydrobenzodipyrans as 5-HT2A/2C receptor probes". Bioorganic & Medicinal Chemistry. 10 (10): 3301–6. doi:10.1016/S0968-0896(02)00209-2. PMID 12150876.
- Schultz DM, Prescher JA, Kidd S, Marona-Lewicka D, Nichols DE, Monte A (June 2008). "'Hybrid' benzofuran-benzopyran congeners as rigid analogs of hallucinogenic phenethylamines". Bioorganic & Medicinal Chemistry. 16 (11): 6242–51. doi:10.1016/j.bmc.2008.04.030. PMC 2601679. PMID 18467103.
- "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" [Regulations Regarding Narcotic Drugs, Psychotropic Substances and Precursors Controlled in Latvia]. Methodological Guidelines for the Application of Annex 1 to the Cabinet Regulation No. 847 (in Latvian). Ministry of Health of the Republic of Latvia. 8 November 2005.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.