Etamsylate
Etamsylate (sometimes spelled ethamsylate) is an antihemorrhagic agent which is believed to work by increasing resistance in the endothelium of capillaries and promoting platelet adhesion.[1][2] It also inhibits biosynthesis and action of those prostaglandins which cause platelet disaggregation, vasodilation and increased capillary permeability.[3]
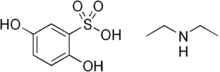 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cyclonamine, Dicynene, Dicynone, Haemostop, Menostat |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.265 |
| Chemical and physical data | |
| Formula | C10H17NO5S |
| Molar mass | 263.31 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Indications
Prophylaxis and control of haemorrhages from small blood vessels, neonatal intraventricular haemorrhage,[4] capillary bleeding of different etiology, including: menorrhagia and metrorrhagia without organic pathology, after trans-urethral resection of the prostate, hematemesis, melena, hematuria, epistaxis; secondary bleeding due to thrombocytopenia or thrombocytopathia, hypocoagulation, prevention of periventricular hemorrhages in prematurely born children.[5]
Mechanism of action
Etamsylate is a haemostatic agent; also promotes angioprotective and proaggregant action. It stimulates thrombopoiesis and their release from bone marrow. Haemostatic action is due to activation of thromboplastin formation on damaged sites of small blood vessels and decrease of PgI2 (Prostacyclin I2) synthesis; it also facilitates platelet aggregation and adhesion, that at last induce decrease and stop of hemorrhage.[6]
The precise mechanism of action of etamsylate is unknown. It has been shown to reduce bleeding time and blood loss from wounds. This appears to relate to increased platelet aggregation mediated by a thromboxane A2 or prostaglandin F2a dependent mechanism. It has also been associated with decreased concentrations of 6-oxoprostaglandin F1a, a stable metabolite of prostacyclin. Prostacyclin is a potent vasodilator, and may be implicated in reperfusion; it is also a disaggregator of platelets. Whereas prostaglandins themselves may have a role in regulating cerebral blood flow, etamsylate appears to have no effect on cerebral blood flow. Etamsylate was also thought to stabilise capillaries, reinforcing capillary membranes by polymerising hyaluronic acid. [7] Etamsylate limits capillary bleeding through its action on hyaluronic acid and initial studies showed a reduction in intraventricular haemorrhage.[8] Etamsylate may also have an effect on the microcirculation, encouraging platelet aggregation and vasoconstriction and therefore haemostasis. It also inhibits the effects of the prostaglandin mediated vasodilatation and increased capillary permeability, thereby reducing oedema secondary to capillary leakage. It is also possible that etamsylate would reduce reperfusion haemorrhage in ischaemic areas of the brain, preventing secondary damage. [1] By inhibiting the effects of prostaglandins, etamsylate may exert an effect by closing the patent ductus and thereby increasing cerebral blood flow.[9]
References
- Schulte J, J; Osborne, J; Benson, JW; Cooke, R; Drayton, M; Murphy, J; Rennie, J; Speidel, B (January 2005). "Developmental outcome of the use of etamsylate for prevention of periventricular haemorrhage in a randomised controlled trial". Arch. Dis. Child. Fetal Neonatal Ed. 90 (1): F31–5. doi:10.1136/adc.2003.035790. PMC 1721806. PMID 15613570.
- Elbourne D, Ayers S, Dellagrammaticas H, Johnson A, Leloup M, Lenoir-Piat S (May 2001). "Randomised controlled trial of prophylactic etamsylate: follow up at 2 years of age". Arch. Dis. Child. Fetal Neonatal Ed. 84 (3): F183–7. doi:10.1136/fn.84.3.F183. PMC 1721248. PMID 11320045.
- Kovács, L; Falkay, G (Nov 15, 1981). "Etamsylate as inhibitor of prostaglandin biosynthesis in pregnant human myometrium in vitro". Experientia. 37 (11): 1182–3. doi:10.1007/BF01989908. PMID 7319004.
- Martindale, The Complete Drug Reference, 36th edition, page: 1065 Drug-Etamsylate
- "Bulgarian Pharmaceutical Group Ltd". Archived from the original on 2012-10-10. Retrieved 2013-08-10.
- "Поиск по базе данных ЛС, опции поиска: МНН — Этамзилат, флаги "Искать в реестре зарегистрированных ЛС", "Искать ТКФС", "Показывать лекформы"". Обращение лекарственных средств. ФГУ «Научный центр экспертизы средств медицинского применения» Росздравнадзора РФ. 2008-03-27. Archived from the original on 22 August 2011. Retrieved 7 April 2008.
- Hunt, RW (January 2005). "Etamsylate for prevention of periventricular haemorrhage" (PDF). Archives of Disease in Childhood: Fetal and Neonatal Edition. 90 (1): F3–5. doi:10.1136/adc.2003.045625. PMC 1721808. PMID 15613569.
- Martindale, The Complete Drug Reference, 36th edition - page 1050
- Perlman, Jeffrey M.; Hill, Alan; Volpe, Joseph J. (1 November 1981). "The effect of patent ductus arteriosus on flow velocity in the anterior cerebral arteries: Ductal steal in the premature newborn infant". The Journal of Pediatrics. 99 (5): 767–771. doi:10.1016/S0022-3476(81)80408-8. PMID 6795324.