PET-MRI
Positron emission tomography–magnetic resonance imaging (PET–MRI) is a hybrid imaging technology that incorporates magnetic resonance imaging (MRI) soft tissue morphological imaging and positron emission tomography (PET) functional imaging.[1]
| Positron emission tomography–magnetic resonance imaging | |
|---|---|
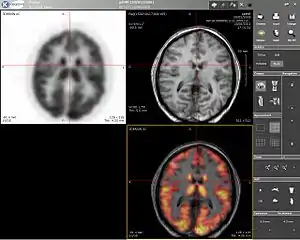
Computer screenshot showing a PET image (upper left), MRI image (upper right) and the combined PET-MRI image where PET data is overlaid over the MRI data (lower right) | |
| Purpose | used in clinical field of oncology |
The combination of PET and MRI was first proposed in 1991 by R. R. Raylman. [2] Simultaneous PET/MR detection was first demonstrated in 1997, however it took another 13 years, and new detector technologies, for clinical systems to become commercially available.[3]
Applications
Presently, the main clinical fields of PET-MRI are oncology,[4][5][6] cardiology,[7] neurology,[8][9][10] and neuroscience.[11] Research studies are actively conducted at the moment to understand benefits of the new PET-MRI diagnostic method. The technology combines the exquisite structural and functional characterization of tissue provided by MRI with the extreme sensitivity of PET imaging of metabolism and tracking of uniquely labeled cell types or cell receptors.
Manufacturers
Several companies offer clinical and pre-clinical combined PET-MR system, clinical systems are available from Philips, Siemens, GE. There are varying approaches to the combination of the two technologies. Some designs are essentially separate machines, in the same room, with a bed that can transfer a patient from one scanner to another.[12][13] Fully integrated systems are the most technically challenging to achieve, but provide greatest benefits in terms of the ability to make simultaneous, exactly aligned, acquisitions.[14][15]
Clinical systems
The first two clinical whole body PET-MRI systems were installed by Philips at Mount Sinai Medical Centre in the United States and at Geneva University Hospital in Switzerland, in 2010. The system featured a PET and MRI scanner separated by a revolving bed.[16][17]
Siemens was the first company to offer simultaneous PET/MR acquisitions, with the first systems installed in 2010 based on avalanche photodiode detectors.[18][3]
On 16th February, 2013,[19] Apollo Hospitals launched the first PET-MRI in South Asia.[20][21]
Currently Siemens and GE are the only companies to offer a fully integrated whole body and simultaneous acquisition PET-MRI system. The Siemens system (Biograph mMR) received a CE mark[22] and FDA approval[23] for customer purchase in 2011.
The GE system (SIGNA PET/MR) received its 510K & CE mark in 2014.
Preclinical systems
Currently, the combination of positron emission tomography (PET) and magnetic resonance imaging (MRI) as a hybrid imaging modality is receiving great attention not only in its emerging clinical applications but also in the preclinical field. Several designs based on several different types of PET detector technology have been developed in recent years, some of which have been used for first preclinical studies.[24][25][26]
Several companies offer MR-compatible preclinical PET scanner inserts for use in the bore of an existing MRI, enabling simultaneous PET/MR image acquisition.[27][28][29][30]
Comparison with PET-CT
The combination of PET with X-ray computed tomography (CT) is the more established PET imaging technology. With both PET-CT and PET-MR the intended advantage is to combine functional imaging provided by PET, with structural(anatomical) information from CT or MRI. Although images from different modalities collected at different scanning sessions can be overlaid by image registration, a simultaneous acquisition offers better alignment of images and direct correlation. Combining imaging modalities in one single scanning session also has the advantage of reducing the number of appointments and therefore improving patient comfort.[31][32]
The same clinical decisions that would influence the choice between stand-alone CT or MR imaging would also determine areas where PET-CT or PET-MR would be preferred.[14] For example, one advantage of MRI compared to CT is its superior soft tissue contrast, while CT has the advantage of being much faster than MRI.
One clear advantage of PET-MR compared to PET-CT is the lower total ionising radiation dose obtained. For body PET-CT applications, the CT part of the examination constitutes approximately 60-80% of the radiation dose, with the remaining radiation dose originating from the PET radiopharmaceutical.[33] In contrast, no ionising radiation dose is obtained from MRI. PET-MR is therefore appealing in children, in particularly for serial follow-up examinations as used in oncology or chronic inflammatory conditions.[34]
Attenuation correction
PET-MRI systems don't offer a direct way to obtain attenuation maps, unlike stand-alone PET or PET-CT systems.[35][36]
Stand alone PET systems' attenuation correction (AC) is based on a transmission scan (mu - map) acquired using a 68Ge (Germanium-68) rotating rod source, which directly measures photon attenuation at 511 keV.[35][37] PET-CT systems use a low-dose CT scan for AC. Since X-rays have a range of energies lower than 511 keV, AC values are closely approximated from Hounsfield units.[38]
There is no correlation between MR image intensity and electron intensity, therefore conversion of MR images into an attenuation map is difficult.[39][35][37] This is an active area of research and a range of approaches have been developed. One method uses a Dixon MRI sequence, and segments the resultant image into fat and water, with pre-set attenuation factors. Disadvantages of this method include a lack of bone attenuation, and loss of the true continuous range of attenuation factors. Comparisons with PET-CT attenuation maps for oncology purposes however have shown that this is a usable technique.[37] The Dixon method can be combined with ultrashort echo time (UTE) sequences to better identify bone and increase the possible classes of tissue for segmentation. More sequences increase MRI acquisition time, and therefore the risk of motion artefacts.[40]
In areas of the body with predictable structures (e.g. the head), segmentation (where tissue is categorised using the MRI image data), or "atlas" methods can be used. In atlas methods a standard MR image, with associated CT attenuation data, can be warped to fit the actual patient anatomy. Disadvantages of this method include difficulty with unusual anatomy, a need for a suitable library of images, and the need to account for MR coil attenuation.[37] Synthetic, or Substitute CT (sCT) methods to generate CT like data from MRI are also of interest for radiotherapy planning, and have been primarily investigated for sites in the head. While some of these use an atlas technique, many take a voxel approach where actual voxel intensities (contrast data) are used in combination with machine learning (trained on MR/CT data) to assign electron density values.[37][41][42]
In many of the above methods, MRI artifacts (e.g. from physiological motion) can affect attenuation correction accuracy.[37][43]
References
- Antoch, Gerald; Bockisch, Andreas (2008). "Combined PET/MRI: a new dimension in whole-body oncology imaging?". European Journal of Nuclear Medicine and Molecular Imaging. 36 (S1): 113–120. doi:10.1007/s00259-008-0951-6. ISSN 1619-7070. PMID 19104802. S2CID 8153201.
- "Reduction of positron range effects by the application of a magnetic field: For use with positron emission tomography". deepblue.lib.umich.edu. hdl:2027.42/128772. Retrieved 2020-11-20.
- Luna, Antonio; Vilanova, Joan C.; Jr, L. Celso Hygino da Cruz; Rossi, Santiago E. (2013). Functional Imaging in Oncology: Biophysical Basis and Technical Approaches. Springer Science & Business Media. p. 421. ISBN 9783642404122.
- Buchbender C; Heusner TA; Lauenstein TC; Bockisch A; et al. (June 2012). "Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis". Journal of Nuclear Medicine. 53 (6): 928–38. doi:10.2967/jnumed.112.105338. PMID 22582048.
- Buchbender C; Heusner TA; Lauenstein TC; Bockisch A; et al. (August 2012). "Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma". Journal of Nuclear Medicine. 53 (8): 1244–52. doi:10.2967/jnumed.112.109306. PMID 22782313.
- Martinez-Möller A; Eiber M; Nekolla SG; et al. (September 2012). "Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology". Journal of Nuclear Medicine. 53 (9): 1415–26. doi:10.2967/jnumed.112.109348. PMID 22879079.
- Rischpler C; Nekolla SG; Dregely I; Schwaiger M (March 2013). "Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects". Journal of Nuclear Medicine. 54 (3): 402–15. doi:10.2967/jnumed.112.105353. PMID 23404088.
- http://www.nih.gov/news/health/sep2011/cc-26.htm%5B%5D
- Dimou E; Booij J; Rodrigues M; et al. (June 2009). "Amyloid PET and MRI in Alzheimer's disease and mild cognitive impairment". Current Alzheimer Research. 6 (3): 312–9. doi:10.2174/156720509788486563. PMID 19519314.
- Bremner JD; Vythilingam M; Vermetten E; et al. (May 2003). "MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder". The American Journal of Psychiatry. 160 (5): 924–32. doi:10.1176/appi.ajp.160.5.924. PMID 12727697.
- Cho, Zang Hee; Son, Young Don; Choi, Eun Jung; Kim, Hang Keun; Kim, Jeong Hee; Lee, Sang Yoon; Ogawa, Seiji; Kim, Young Bo (3 August 2012). "In-vivo human brain molecular imaging with a brain-dedicated PET/MRI system". Magnetic Resonance Materials in Physics, Biology and Medicine. 26 (1): 71–79. doi:10.1007/s10334-012-0329-4. PMID 22864642. S2CID 10721235.
- Torigian, Drew A.; Zaidi, Habib; Kwee, Thomas C.; Saboury, Babak; Udupa, Jayaram K.; Cho, Zang-Hee; Alavi, Abass (April 2013). "PET/MR Imaging: Technical Aspects and Potential Clinical Applications". Radiology. 267 (1): 26–44. doi:10.1148/radiol.13121038. PMID 23525716.
- "The Past, Present and Future of PET/MRI Scanners". Imaging Technology News. 5 May 2017. Retrieved 15 January 2019.
- Jadvar, Hossein; Colletti, Patrick M. (January 2014). "Competitive advantage of PET/MRI". European Journal of Radiology. 83 (1): 84–94. doi:10.1016/j.ejrad.2013.05.028. PMC 3800216. PMID 23791129.
- Mannheim, Julia G.; Schmid, Andreas M.; Schwenck, Johannes; Katiyar, Prateek; Herfert, Kristina; Pichler, Bernd J.; Disselhorst, Jonathan A. (July 2018). "PET/MRI Hybrid Systems". Seminars in Nuclear Medicine. 48 (4): 332–347. doi:10.1053/j.semnuclmed.2018.02.011. PMID 29852943.
- Wood, Harry (28 May 2010). "PET-MRI scanner opens new frontier in medical imaging". Medical Technology Business Europe. Retrieved 15 January 2019.
- Muzic, Raymond F.; DiFilippo, Frank P. (July 2014). "Positron Emission Tomography-Magnetic Resonance Imaging: Technical Review". Seminars in Roentgenology. 49 (3): 242–254. doi:10.1053/j.ro.2014.10.001. PMC 4451572. PMID 25497909.
- Zaidi, Habib (2016). PET/MRI: Advances in Instrumentation and Quantitative Procedures, An Issue of PET Clinics. Elsevier Health Sciences. ISBN 9780323417686.
- Group, Apollo Hospitals. "Smt. Sheila Dikshit Unveils South Asia's First PET SUITE at Indraprastha Apollo Hospitals". www.prnewswire.com. Retrieved 2020-01-04.
- "South Asia's first PET-MR SUITE at Indraprastha Apollo Hospitals !". Apollo Hospitals. Retrieved 2020-01-04.
- "India's First PET MRI Scan Center in Delhi | Cost, Price | PET-MRI". Retrieved 2020-01-04.
- "Siemens receives CE mark for whole-body molecular MR system". Healthcare Sector, Siemens AG. 2011-06-01. Retrieved 2014-01-05.
- "FDA clears new system to perform simultaneous PET, MRI scans". U.S. Food and Drug Administration. 2011-06-10. Retrieved 2014-01-04.
- Judenhofer, Martin S.; Cherry, Simon R. (2013). "Applications for Preclinical PET/MRI". Seminars in Nuclear Medicine. 43 (1): 19–29. doi:10.1053/j.semnuclmed.2012.08.004. PMID 23178086.
- Schulz, Volkmar; Weissler, Bjoern; Gebhardt, Pierre; Solf, Torsten; Lerche, Christoph; Fischer, Peter; Ritzert, Michael; Piemonte, Claudio; Goldschmidt, Benjamin; Vandenberghe, Stefaan; Salomon, Andre; Schaeffter, Tobias; Marsden, Paul (2011). SiPM based preclinical PET/MR insert for a human 3T MR: first imaging experiments. Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 IEEE. pp. 4467–4469. doi:10.1109/NSSMIC.2011.6152496. ISBN 978-1-4673-0120-6. S2CID 27832030.
- Wehner, Jakob; Weissler, Bjoern; Dueppenbecker, Peter; Gebhardt, Pierre; Schug, David; Ruetten, Walter; Kiessling, Fabian; Schulz, Volkmar (2013). "PET/MRI insert using digital SiPMs: Investigation of MR-compatibility". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 734 (Pt B): 116–121. Bibcode:2014NIMPA.734..116W. doi:10.1016/j.nima.2013.08.077. PMC 4376059. PMID 25843999.
- Omidvari, Negar; Cabello, Jorge; Topping, Geoffrey; Schneider, Florian Roland; Paul, Stephan; Schwaiger, Markus; Ziegler, Sibylle I (4 October 2017). "PET performance evaluation of MADPET4: a small animal PET insert for a 7-Tesla MRI scanner". Physics in Medicine and Biology. 62 (22): 8671–8692. doi:10.1088/1361-6560/aa910d. PMID 28976912.
- Wehner, J; Weissler, B; Dueppenbecker, P M; Gebhardt, P; Goldschmidt, B; Schug, D; Kiessling, F; Schulz, V (21 March 2015). "MR-compatibility assessment of the first preclinical PET-MRI insert equipped with digital silicon photomultipliers". Physics in Medicine and Biology. 60 (6): 2231–2255. Bibcode:2015PMB....60.2231W. doi:10.1088/0031-9155/60/6/2231. PMID 25684065.
- Goldenberg, Joshua M.; Cárdenas-Rodríguez, Julio; Pagel, Mark D. (26 January 2018). "Preliminary Results that Assess Metformin Treatment in a Preclinical Model of Pancreatic Cancer Using Simultaneous [18F]FDG PET and acidoCEST MRI". Molecular Imaging and Biology. 20 (4): 575–583. doi:10.1007/s11307-018-1164-4. PMC 6043393. PMID 29374343.
- Nagy, Kálmán; Tóth, Miklós; Major, Péter; Patay, Győző; Egri, G.; Häggkvist, Jenny; Varrone, Andrea; Farde, Lars; Halldin, Christer; Gulyás, Balázs (2013). "Performance Evaluation of the Small-Animal nanoScan PET/MRI System". Journal of Nuclear Medicine. 54 (10): 1825–1832. doi:10.2967/jnumed.112.119065. PMID 23990683.
- Kaplan, Deborah Abrams (12 June 2013). "PET/MRI: Reflections Two Years After FDA Approval". Diagnostic Imaging. Retrieved 15 January 2019.
- Pichler BJ, Wehrl HF, Kolb A, Judenhofer MS (2008). "Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging?". Semin Nucl Med. 38 (3): 199–208. doi:10.1053/j.semnuclmed.2008.02.001. PMC 2762705. PMID 18396179.
- Martí-Climent, Josep M.; Prieto, Elena; Morán, Verónica; Sancho, Lidia; Rodríguez-Fraile, Macarena; Arbizu, Javier; García-Velloso, María J.; Richter, José A. (December 2017). "Effective dose estimation for oncological and neurological PET/CT procedures". EJNMMI Research. 7 (1): 37. doi:10.1186/s13550-017-0272-5. ISSN 2191-219X. PMC 5403773. PMID 28439843.
- Ehman, Eric C.; Johnson, Geoffrey B.; Villanueva-Meyer, Javier E.; Cha, Soonmee; Leynes, Andrew Palmera; Larson, Peder Eric Zufall; Hope, Thomas A. (November 2017). "PET/MRI: Where might it replace PET/CT?". Journal of Magnetic Resonance Imaging. 46 (5): 1247–1262. doi:10.1002/jmri.25711. PMC 5623147. PMID 28370695.
- Keereman, Vincent; Mollet, Pieter; Berker, Yannick; Schulz, Volkmar; Vandenberghe, Stefaan (2013-02-01). "Challenges and current methods for attenuation correction in PET/MR". Magnetic Resonance Materials in Physics, Biology and Medicine. 26 (1): 81–98. doi:10.1007/s10334-012-0334-7. ISSN 0968-5243. PMID 22875599. S2CID 22198626.
- van Dalen, Jorn A.; Visser, Eric P.; Vogel, Wouter V.; Corstens, Frans H. M.; Oyen, Wim J. G. (2007-03-01). "Impact of Ge-68∕Ga-68-based versus CT-based attenuation correction on PET". Medical Physics. 34 (3): 889–897. Bibcode:2007MedPh..34..889V. doi:10.1118/1.2437283. ISSN 2473-4209. PMID 17441234.
- Wagenknecht, Gudrun; Kaiser, Hans-Jürgen; Mottaghy, Felix M.; Herzog, Hans (2013-02-01). "MRI for attenuation correction in PET: methods and challenges". Magnetic Resonance Materials in Physics, Biology and Medicine. 26 (1): 99–113. doi:10.1007/s10334-012-0353-4. ISSN 0968-5243. PMC 3572388. PMID 23179594.
- Bai, Chuanyong; Shao, Ling; Silva, A. J. Da; Zhao, Zuo (October 2003). "A generalized model for the conversion from CT numbers to linear attenuation coefficients". IEEE Transactions on Nuclear Science. 50 (5): 1510–1515. Bibcode:2003ITNS...50.1510B. doi:10.1109/tns.2003.817281. ISSN 0018-9499.
- Hofmann, Matthias; Pichler, Bernd; Schölkopf, Bernhard; Beyer, Thomas (2009-03-01). "Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques". European Journal of Nuclear Medicine and Molecular Imaging. 36 (1): 93–104. doi:10.1007/s00259-008-1007-7. ISSN 1619-7070. PMID 19104810.
- Vandenberghe, Stefaan; Marsden, Paul K (21 February 2015). "PET-MRI: a review of challenges and solutions in the development of integrated multimodality imaging". Physics in Medicine and Biology. 60 (4): R115–R154. arXiv:1510.04875. Bibcode:2015PMB....60R.115V. doi:10.1088/0031-9155/60/4/R115. PMID 25650582.
- Edmund, Jens M.; Nyholm, Tufve (26 January 2017). "A review of substitute CT generation for MRI-only radiation therapy". Radiation Oncology. 12 (1): 28. doi:10.1186/s13014-016-0747-y. PMC 5270229. PMID 28126030.
- Larsson, Anne; Johansson, Adam; Axelsson, Jan; Nyholm, Tufve; Asklund, Thomas; Riklund, Katrine; Karlsson, Mikael (7 September 2012). "Evaluation of an attenuation correction method for PET/MR imaging of the head based on substitute CT images". Magnetic Resonance Materials in Physics, Biology and Medicine. 26 (1): 127–136. doi:10.1007/s10334-012-0339-2. PMID 22955943. S2CID 7334804.
- Hofmann, Matthias; Pichler, Bernd; Schölkopf, Bernhard; Beyer, Thomas (23 December 2008). "Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques". European Journal of Nuclear Medicine and Molecular Imaging. 36 (S1): 93–104. doi:10.1007/s00259-008-1007-7. PMID 19104810.