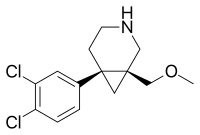
GSK1360707F
GSK1360707F is a potent and selective triple reuptake inhibitor.[1] structurally related to amitifadine and NS-2359 (GSK-372,475).
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C14H17Cl2NO |
| Molar mass | 286.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Synthesis
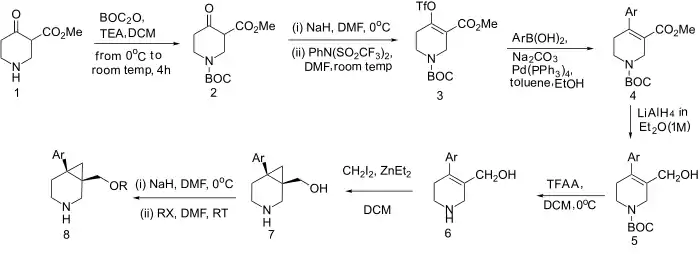
Synthesis of GSK1360707F: WO 2008031772
- BOC Protecting group.
- Enolization and trapping with triflate group (cf Comins' reagent).
- Suzuki reaction
- Reduction (only 1 mol eq. LAH because N-BOC can be reduced to N-Me)
- Trifluoroacetic acid (TFA) removal of protecting group.
- Simmons–Smith reaction cyclopropanation.
- Williamson ether synthesis (c.f. NS patents & paxil).
Transporter occupancy
GSK1360707F has recently (2013) been tested on baboons (Papio anubis) & humans for transporter occupancy using PET.[2]
until recently was under development for the treatment of major depressive disorder; its development was put on hold for strategic reasons.
References
- Micheli F, Cavanni P, Andreotti D, Arban R, Benedetti R, Bertani B, et al. (July 2010). "6-(3,4-dichlorophenyl)-1-[(methyloxy)methyl]-3-azabicyclo[4.1.0]heptane: a new potent and selective triple reuptake inhibitor". Journal of Medicinal Chemistry. 53 (13): 4989–5001. doi:10.1021/jm100481d. PMID 20527970.
- Comley RA, Salinas CA, Slifstein M, Petrone M, Marzano C, Bennacef I, et al. (August 2013). "Monoamine transporter occupancy of a novel triple reuptake inhibitor in baboons and humans using positron emission tomography". The Journal of Pharmacology and Experimental Therapeutics. 346 (2): 311–7. doi:10.1124/jpet.112.202895. PMID 23685546.
| α1 |
| ||||
|---|---|---|---|---|---|
| α2 |
| ||||
| β |
| ||||
| See also | |||||
| D1-like |
| ||||
|---|---|---|---|---|---|
| D2-like |
| ||||
| |||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.